Werner wins DARPA Young Faculty Award to make material that captures CO2
By Patrick L. Kennedy
Dozing in the classroom? That droopiness might not be due to the quality of the lecture, says Assistant Professor Joerg Werner (ME, MSE). The culprit could be carbon dioxide. While the amount of CO2 in atmospheric (outdoor) air is problematic enough at 0.04%, in an enclosed space it can rise to above 0.1%, affecting humans’ focus, productivity, and even health.
If that’s the case in an ordinary building with windows or vents providing some access to fresh air, imagine the situation in a submarine, an underground bunker, or a spacecraft. This is what concerns the U.S. Defense Advanced Research Projects Agency (DARPA). Werner has won a highly competitive DARPA Young Faculty Award to help solve the problem with a novel material that would capture carbon dioxide in a targeted and efficient way.

“Especially for a space station, you need a very small, lightweight air purification system that uses very little energy,” Werner says. “I apply my chemistry training to make new materials. In this case, we’re trying to make new, ultra-thin polymer coatings that can capture CO2 very efficiently.”
In previous projects, Werner has collaborated with Associate Professor Keith Brown (ME, MSE, Physics) on thin electrochemical films for high-capacity battery technology. They created these films with the help of an automated experimenter that they built, similar to Brown’s BEAR system. Capable of making and analyzing hundreds of films in a matter of hours, the tool will be useful in Werner’s DARPA project as well.
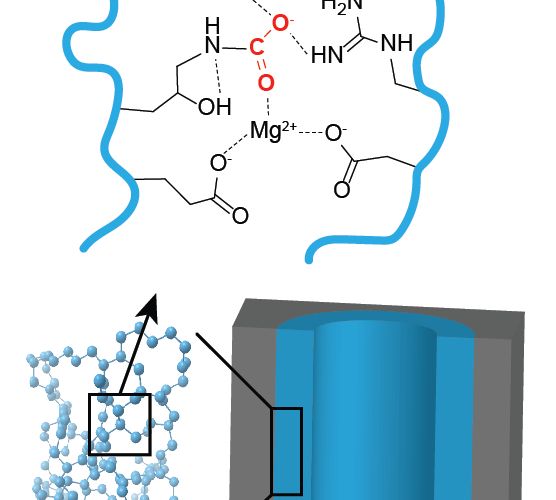
The air purification system—essentially, a filter—will combine two new materials. The ultra-thin polymer coating that absorbs CO2 will be layered atop a porous sorbent—a kind of sponge. The sorbent will be pockmarked with pores that plenty of carbon dioxide can get caught in. Inspired by the lungs, the architecture of the filter will ensure fast air flow and efficient capture of the CO2 in a relatively small package.
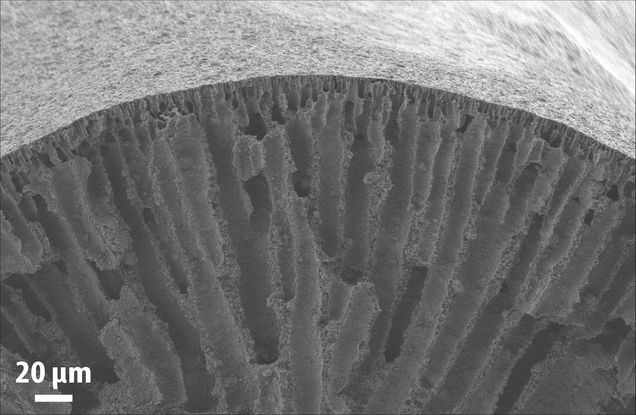
The project comes with some challenges, says Werner. One is ensuring the polymer doesn’t also absorb desirable molecules like oxygen and nitrogen. At the same time, it shouldn’t bind the CO2 too strongly, since DARPA would like to extract the gas later to efficiently regenerate the sorbents for reuse and potentially as a feedstock for microbes that will make other stuff needed in space or undersea.
To attain this Goldilocks material, the researchers will need to make and test thousands of combinations of chemical groups with the help of Brown’s automated system and a novel method of electrodeposition—normally done with metals in jewelry making and other industries. “We figured out a way to use that kind of process, but for polymers,” Werner says.
Eventually, Werner hopes to work with fellow members of the larger BU Energy & Sustainable Technologies Lab to apply the technology to other decarbonization efforts.
“I would be interested in exploring how this could translate even to the atmospheric capture of CO2, if it could be done efficiently” Werner says. “That’s well beyond the scope of this award, but it’s a very important topic and will continue to be so.”