BU’s Core Organoid Research Spans CRC & MED Campuses to Restore Organ Function
By Danny Giancioppo and Jack Osmond | Photos Supplied by Martin Thunemann, Ella Zeldich, and Ben Wolozin
Organoids are a growing trend in biomedical research fields internationally––but what are they? As the name suggests, it’s a simplified model of an organ made up of cells, studied both in vitro (outside a living organism) and in vivo (inside a living organism) to enhance the understanding and treatment of organ-related diseases and disorders. Their pathology, phenotype, and––in the future––repair, are paths made clearer for researchers by utilizing these human stem cells to recreate miniature brains, pancreases, and other existing organ functionality on a cellular scale.
At Boston University, organoid research extends beyond even a single campus. On the Charles River Campus, faculty members of the Neurophotonics Center have been diligently working with faculty of the Medical Campus to further one another’s work, and better the understanding of neurological subjects such as Alzheimer’s, Down Syndrome, and Parkinson’s, as well as Primary Liver Cancer and Diabetes. The core of organoid-related research at Boston University, then, is one which insists upon cross-campus collaboration to further the application and impact of organ treatment and understanding.
But what does each core facet of organoid research at BU look like, and how do they overlap? To see the convergent work produced across the two campuses, it is imperative to first understand what each core function of organoid research looks like, and the faculty who lead it.
Creating Organoids:
Neurodegeneration: Ben Wolozin’s Aim to Understand Alzheimer’s and ALS
Neurodegenerative disease is a field as complex as the human brain itself. The use of organoids in such research suggests a fervor for not only learning the mechanisms of neurodegeneration, but a willingness to peer further into the brain than we can with the naked eye. You’d have to be pretty dedicated, then, to delve so deep into the why and how of neurodegeneration. Ask Ben Wolozin, MD, PhD, and he’ll tell you he’s the man for the job.
“I’m a ‘neurodegenerate,’” he jokes, explaining that his dedication to the study of neurodegenerative disease leaves him as something of an “intellectual tourist” among other fields and subsections of neuroscience. No matter the source, so long as the research is well-founded, Wolozin follows the work to enhance his studies of what causes brain function to falter and fluctuate. Ultimately, this work identifies him as a neuronal cell biologist.
Wolozin explains, when asked what use organoids have toward understanding the nature of such diseases: “In the context of neurodegeneration, people in the field of Alzheimer’s disease and ALS have had a lot of difficulty developing stem cells to show pathology. You can see disease effects, kind of, but you just don’t see the stuff you see in the brain.”
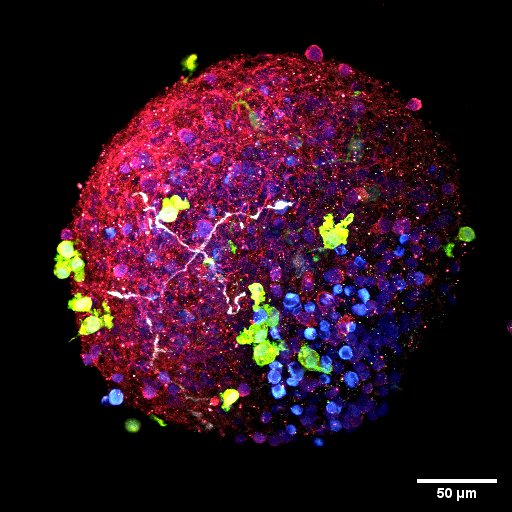
“[With] organoids, you start with some kind of neural precursor cells, and you grow them floating in culture and let them grow up over nine months. And they recapitulate a lot of the cortical structures that you see [in the brain]. They develop some of the types of the excitatory cells, and particularly once they’ve been in culture for a while, they start to develop astrocytes. In a way, it kind of recapitulates what happens in a fetus, as it takes nine months!”
This comparison to human reproduction is just one of the human elements that makes organoid research so appealing, not only in neurodegenerative research, but beyond. What increasingly drives people toward organoids is their close-knit connection, in fact their origin, from human cells. “If you want to know if it happens in people, you want to be able to look in a human system,” Dr. Wolozin says.
Such a direct line to the human cellular system can more easily mimic the functionality, or pathology, of not only nominal brain activity and evolution, but malfunctioning cells. You can see the effect in various models as well as in human neurons. This means you can also get diseased cells, or those with genetic variations and mutations to “isogenically” correct said mutations in the organoid. This proves invaluable toward understanding the causes of diseases like Alzheimer’s, in that you can test the organoid to see if what is in the gene may be causing the disease.
This sort of mirroring of neurodegenerative pathology is in large part what drives the collaborative nature of Dr. Wolozin’s work. “This actually began as a cross-collaborative work that I actually put in with Christine Cheng to get a Kilachand Award.” Although Professor Cheng has since left BU’s campus, the collaborative nature of Wolozin’s work has continued. “I have also interacted with three different groups explicitly about this. I’ve talked with Anna Devor, and we actually did some experiments looking at these things growing in mouse brains. I didn’t have a great question for myself, but Julia TCW is now doing very similar things with good results. I have worked with Ji-Xin Cheng on using optical methods in noninvasive ways to see the pathology, and that’s ongoing work that we’re doing together.”
As for the next stage of organoid research, there are “many, many advancements” to be made, according to Dr. Wolozin. For example, “in Alzheimer’s disease, and a lot of diseases of aging, old people have something that you don’t have––you young whippersnappers. That is cerebrovascular disease. Their blood vessels are clogged. And it’s very clear that diseases of aging are strongly modified by whether or not you get blood to your brain. And so the field is super interested in looking at the interaction between organoids and blood vessels.”
His collaborative work with Anna Devor was one such example of pursuing this line of research––and they’re not alone. More and more members of the field are following suit in their studies by placing organoids on artificial blood vessels or into the brain with the specific focus of blood vessel interaction. In so doing, researchers are growing the capacity and possibility for a more in-depth understanding of how the brain works, and how it doesn’t.
“As we evolve, we’re going to make these things more mature,” Dr. Wolozin explains. “These things improve dramatically with time.”
Neurodevelopment: How Ella Zeldich Studies Neuronal Functionality in Down Syndrome
Ella Zeldich, Assistant Professor at the Chobanian & Avedisian School of Medicine, is using cortical (brain) organoids to research the neurodevelopmental processes associated with Down Syndrome. The genetic condition affects about 1 in 700 babies born, or approximately 200,000 adults in the United States. Also known as trisomy 21, Down Syndrome is caused by a triplication of the 21st chromosome, which leads to pathological changes in the brains of people with the disorder and the development of Alzheimer’s disease earlier than in the general population.
According to Zeldich, studying the pathology of Down Syndrome is particularly difficult because it is both a neurodevelopmental and neurodegenerative disorder. It’s almost like the question of the chicken and the egg––do neurodevelopmental phenotypes lead to neurodegenerative changes, or vice-versa?
“It is well known that there is an extra dosage of amyloid precursor protein in Down Syndrome, as the gene encoding for this protein is located on chromosome 21. The amyloid precursor protein is a paternal protein for a toxic amyloid beta that accumulates in the brain of patients with Alzheimer’s disease. However, we don’t know, to what extent early accumulation of amyloid beta due to trisomy, leads to the different neurodevelopmental phenotypes and dysregulation in neuronal functions,” Zeldich explains, “or, to what extent abnormal neurodevelopmental processes predispose brain cells in Down Syndrome to neurodegeneration and Alzheimer’s related pathology later on.
Zeldich and her research team employ a cortical organoid platform to help answer these questions. The team utilizes induced pluripotent stem cells (iPSCs) to create two types of organoids, one with trisomy 21 and one control to understand the effects of trisomy 21 on the phenotypic and functional properties of certain brain cells. From this, her team has gleaned “some very exciting findings.”

“If we compare the trisomic organoids which have this [extra copy of the] 21st chromosome with the control organoids, we see that the trisomic organoids are smaller, and that there is an underproduction of neurons from deep and superficial cortical layers.”
“Our next goal would be to say, okay, we see these structural and phenotypic changes, and now, we are moving further by trying to recapitulate functional changes in the trisomic neurons in our organoid system.”
Zeldich strongly believes that collaboration is one of the main keys to scientific advancement. “Our current projects through our collaborations are very exciting.” She recently collaborated with Dr. Christopher Gabel, Associate Professor at both the Neurophotonics Center and School of Medicine. The two used calcium imaging to find differences in neuronal activity between trisomic and control organoids. The changes they found correspond to the abnormal neuronal activity observed in the Down Syndrome brain. Zeldich and Gabel plan to expand this collaboration to further their understanding of these variations.
Granted, organoids have their own setbacks. Organoids lack vasculature, limiting their supply of oxygen and nutrients. In addition, the cells within the organoids can only mature to a certain point. To overcome these challenges, Zeldich has recently begun working with Martin Thunemann, Research Assistant Professor of Biomedical Engineering and Anna Devor, Professor of Biomedical Engineering.
Devor and Thunemann have developed an amazing platform where human organoids can be engrafted into mouse brains to overcome some of these “constant challenges.” Zeldich and Thunemann are harnessing this system to integrate Down Syndrome organoids into mouse brains in order to investigate the dysregulation in neuronal activity in an environment that promotes the maturation of neurons populating organoids. “This platform is truly remarkable as it enables us to address questions related to human disease modeling in a functionally and physiologically relevant environment,” Zeldich says. “The end goal is to understand the mechanisms underlying pathological processes. Once we understand these mechanisms we can come up with therapeutic approaches.”
But the research doesn’t stop there. Dr. Zeldich is also using organoids to develop therapeutic interventions for Down Syndrome. In one collaborative study with Professor Dr. Tara Moore and Associate Professor Dr. Maria Medalla of the School of Medicine, Zeldich’s team was able to harness the beneficial properties of extracellular vesicles (tiny particles responsible for intercellular communication) derived from the bone marrow of a monkey. By exposing the organoids to these vesicles, “we saw that some of the neurodevelopmental changes were resolved, and we detected decreased production of amyloid beta in trisomic organoids,” Zeldich explains. In short, organoids may be used to develop therapeutic interventions to alleviate some of the pathological changes related to Down Syndrome.
Ella Zeldich is hopeful for the future of organoids, and doesn’t see their great progress slowing down any time soon. “These [organoid] systems can only expand. More diseases will be modeled – we are a long way from the limit.”
Implementing Organoids:
Xenografts: Martin Thunemann’s Collective Efforts to Understand Disease
“Everybody sees that it works,” says Martin Thunemann, PhD, Research Assistant Professor of BME, on the subject of organoids. “You can take brain organoids, implant them into mice, and see that something is happening. I think with our previous work, we are at a stage where we know the right way of doing these implantations, and that it works, and now we want to make it better and more meaningful.”

This has driven Professor Thunemann to thoroughly advance the study of organoid research at Boston University. Through the use of xenografts––tissue, or in this case, brain organoids, which are grown from human cells and implanted into mice brains––Dr. Thunemann has been able to pool together the in-house collection of organoids generated at BU (and beyond, through contacts at UC San Diego) from faculty such as Dr. Zeldich, Dr. Wolozin, and Dr. Xue Han. Together, they are greatly enhancing the understanding and potential application of brain organoids in neurodegenerative and developmental disorders.
“I see convergence in enabling disease-relevant models generated and studied at [the] MED campus being investigated with highly sophisticated state-of-the-art methods developed, optimized, and used at [the] CRC.”
In the case of on-campus faculty, Dr. Thunemann and his research team work closely with colleagues to further both organoid designers’ (Wolozin, Han, Zeldich, etc.) and implanters’ (Thunemann and Devor, et al.) understanding of human-based cells. Where other faculty deal more with the curation of organoids and assembloids, Dr. Thunemann implants them into immunodeficient mice brains––therefore assuring the human cells aren’t eliminated by the mouse’s immune system––and studies how the organoids interact with, and in some cases are integrated with the naturally occurring mouse brain cells and present disease phenotypes in the brain and other tissues. Anna Devor, PhD, Professor of BME, also follows this style of understanding the connectivity between organoids and host brain cells, specifically through blood vessel interactions. Her and Dr. Thunemann’s work proves to be therefore symbiotic in advancing the field.

“We are glad to build on Dr. Devor’s experience in the field of neurovascular interactions and functional brain imaging to improve our xenograft approach,” Dr. Thunemann explains. “One important aspect of our model is that blood vessels from the mouse grow into the organoid and provide it with oxygen and nutrients.” Essentially, integrating the organoid into the brain as though it were a part of it. And when the cells are accepted as a part of the larger organ, Thunemann, Devor, et al. can see how a disease might develop in said organoid, and why. “In the future, we hope that this model can help to investigate disease phenotypes that affect both brain tissue and brain vasculature, including, for example, neurodegenerative disorders such as Alzheimer’s.”
But that’s not all. While in preliminary stages, the opportunity for cell replacement therapy allows not only the chance to understand phenotypes and origins of diseases, but perhaps even means to treat them. “The question is at which point this can move from single first-in-human trials to something which can be done more routinely. I’m hesitant to say for the brain this will be very easy, because it’s a very, very complicated organ, which not only requires the cells to be present but [to be] connected the right way, so they can contribute meaningfully. If I had to venture a guess, I think the first in-human studies would be on something like the pancreas. You have Type-1 diabetes patients, and you can give them their own pancreatic beta cells made from their own stem cells back, and hopefully have them not need to take insulin anymore.”
Biomaterials: Boosting Organoid Growth
To guide the way these organoids assimilate with other cells, Timothy O’Shea, PhD, has provided developmental boosts to Drs. Thunemann and Devor by way of biomaterials, which are layered above or directly beneath the organoids when placed in mice.
“He uses biomaterials in combination with other types of neurogenic stem cells, or neuron precursors, and is looking into, for example, spinal cord injuries,” Dr. Thunemann explains. “Biomaterial can be loaded with molecules acting as developmental cues (or “morphogens”) that affect neuronal growth and maturation and are present during normal neurodevelopment.”
In other words, they provide direction for cell growth and development in what would naturally occur during prenatal stages of organ growth. Dr. Thunemann and team hope layering the biomaterials with organoids might essentially kickstart a more natural growth of these newly implanted cells (organoids) as though they were part of the initial developmental process and catching up to the rest of the organ. The use of biomaterials furthermore assists in mitigating the variability of organoid development that can otherwise lead to the onset of unguided growth or a general failure to develop.
Assisting in this project is Kate Herrema, who recently won the Neurophotonics Center’s CAN DO award for the aim of advancing cortical organoid studies. Specifically, she plans to advance the maturation of organoids, lowering risk of failing or uncontrolled development.
“Kate is very special,” Anna Devor explains. “She thinks and writes at the level of an assistant professor if not higher. She is a natural lead investigator. It’s not that she is helping us, it’s we who assist her!
Working alongside Ella Zeldich, Anna Devor, Martin Thunemann, and Timothy O’Shea––in what Dr. Devor describes as a fully collaborative and equally shared effort––Kate’s work is the focal point of BU’s convergent organoid studies. Bringing together the collective efforts of the MED and CRC faculty, Kate’s efforts leverage the many strengths Boston University has to offer and assures a bright and productive future for organoid studies and utility, not only on campus, but the biomedical field at large.
“Life is short, and the only way to get more done is to team up with colleagues and friends to create something much bigger than the sum of its parts,” Dr. Devor says. “We are so fortunate here at BU [in that] we are surrounded by brilliant experts in a wide range of biomedical domains. This is priceless.”