NIH R01 awarded to advance a “Computational Miniature Mesoscope”
 Congratulations to Professor Lei Tian and his co-investigators Ian Davison and David Boas for being awarded a 5-year NIH R01 titled “Computational Miniature Mesoscope for Cortex-wide, Cellular resolution Ca2+ Imaging in Freely Behaving Mice.”
Congratulations to Professor Lei Tian and his co-investigators Ian Davison and David Boas for being awarded a 5-year NIH R01 titled “Computational Miniature Mesoscope for Cortex-wide, Cellular resolution Ca2+ Imaging in Freely Behaving Mice.”
This award evolved from the early days of the BU miniature microscope designed to measure brain function in freely moving and freely singing song birds developed by former BU Professor Tim Gardner and his team. Professor Ian Davison was adapting this miniscope for mouse recordings and was trying to increase the imaging field of view from about 1 mm to 3 mm. This presents quite a challenge when using conventional optics and trying to maintain a small and light-weight package that can be worn on the head of a mouse. Professor Lei Tian had a potential unconventional solution using computational imaging methods. And thus the start of a great new collaboration was born. Tian and Davison were awarded a College of Engineering Dean’s Catalyst Award to initiate their collaboration. They leverage that to get a 2-year NIH R21 award to develop the idea further. And with the work of their exceptional PhD student Yujia Xue (who just successfully defended his PhD, congrats!), they just turned that into a 5-year NIH R01. Great work! The NPC community looks forward to following their great work and learning of the important new discoveries it will provide. The abstract of the grant follows.
ABSTRACT
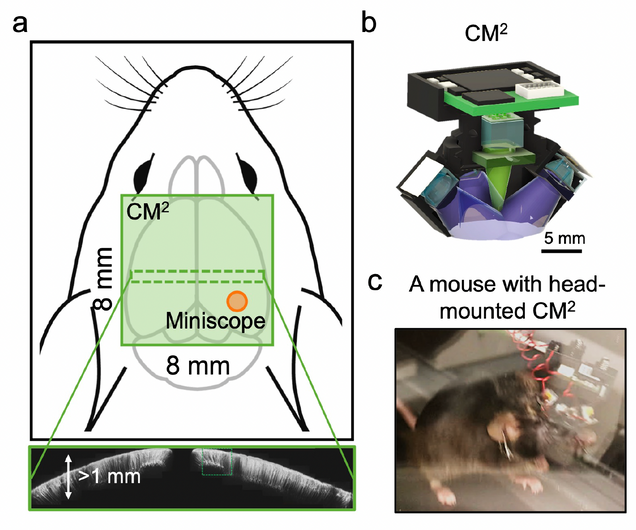
Perception and cognition arise from the coordinated activity of large networks of neurons spanning diverse brain areas. Understanding their emergent behavior requires large-scale activity measurements both within and across regions, ideally at single cell resolution. An integrative understanding of brain dynamics requires cellular-scale data across sensory, motor, and executive areas spanning more than a centimeter. In addition, functional interactions between brain areas vary with motivational state and behavioral goals, making data from freely moving animals particularly critical. Thus, a key goal is the ability to measure activity across the full extent of cortex at cellular resolution as animals engage in complex, cognitively demanding behaviors. However, conventional fluorescence microscopy techniques cannot meet the joint requirements of FOV, resolution, and miniaturization. Here, we propose a Computational Miniature Mesoscope (CM2) that will enable cortex-wide, cellular resolution Ca2+ imaging in freely behaving mice. The premise is that computational imaging leverages advanced algorithms to overcome limitations of conventional optics and significantly expand imaging capabilities. In our proof-of-principle system, we demonstrated single-shot 3D imaging across an 8x7mm2 FOV and 7µm resolution in scattering phantoms (Sci. Adv. 2020), and achieved single-cell resolution on histological sections. Our wearable prototype has now demonstrated visualization of sensory-driven neural activity across the 4x4mm2 main olfactory bulb in both head-fixed and freely moving mice. In this project we will: (Aim 1) advance CM2 hardware to achieve cortex-wide cellular resolution imaging. We will validate the hardware improvement on both phantoms and in vivo experiments. (Aim 2) Develop scattering-informed deep learning for fast and robust recovery of neural signals. We will validate the algorithm on in vivo experiments and benchmark against tabletop 1P and 2P measurements. (Aim 3) Cortex-wide, cellular-resolution Ca2+ imaging during social recognition in freely behaving mice. We will use CM2 to investigate the cross-area, network-scale activity dynamics that guide social interactions between familiar partners – one of the most integrative, multi-sensory, and cognitively demanding forms of neural processing. IMPACT ON PUBLIC HEALTH: This work will establish powerful enabling technology that greatly expands the scale of activity measurements possible in behaving animals, providing access to a wide range of questions about distributed cortical function. As a focused application, we will test the neural signatures of individual recognition during social behavior. We anticipate that our approach can be extended to a broader range of biological questions such as navigation, short- and long-term memory storage, and can potentially lead to new strategies for characterizing the disruptions in neural function that occur in psychiatric disease and neurodegenerative disorders.