COVID Vaccines for Kids Ages 5 to 11: Answers to Common Questions
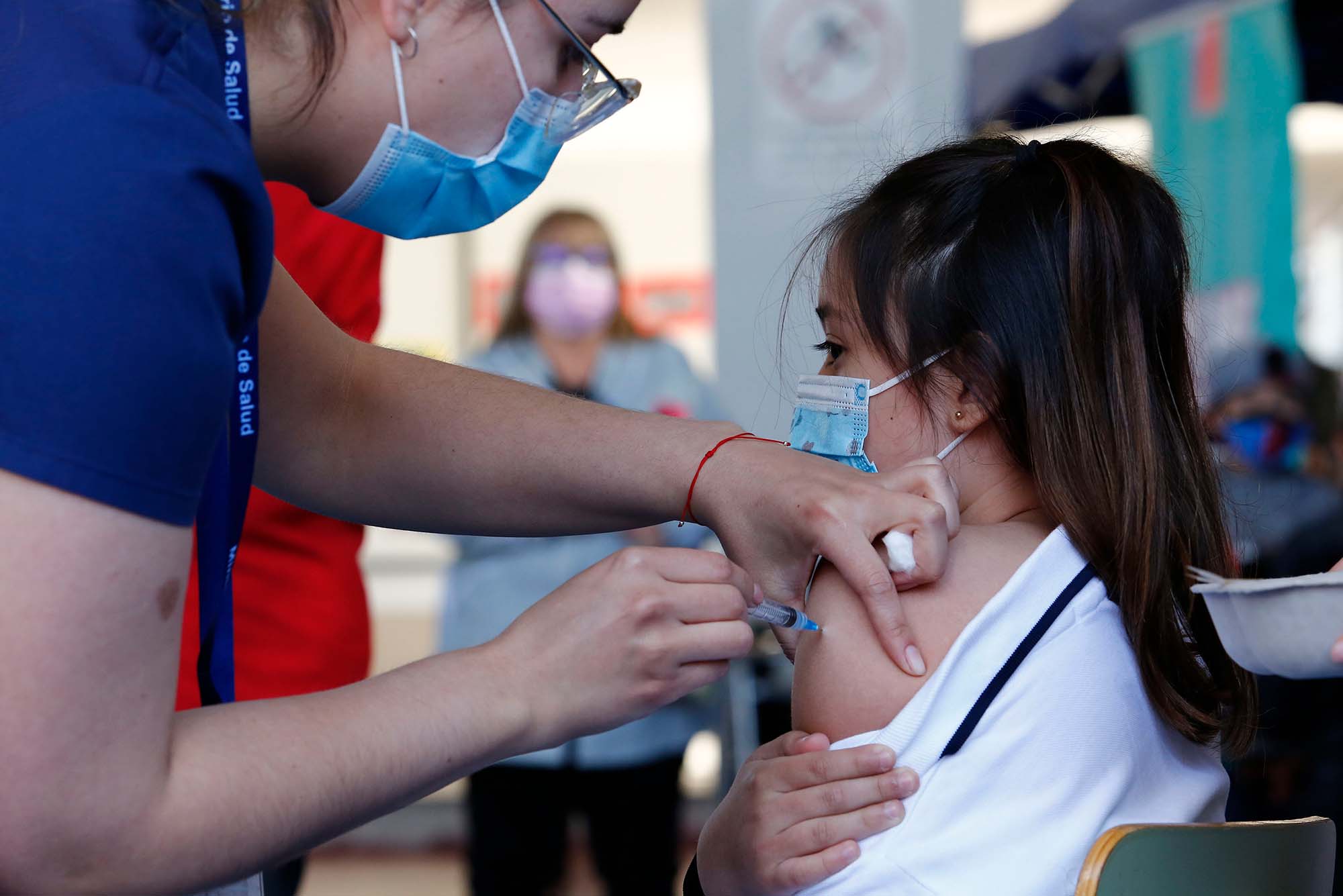
In Ecuador, a health worker administers the first dose of Sinovac vaccine to a student as part of the immunization plan against COVID-19 for children aged 6 to 11 at Alicante del Rosal school on October 1, 2021. In the United States, White House officials announced on October 20 that the FDA and CDC are expected to soon approve and recommend scaled-down Pfizer-BioNTech COVID vaccines for American children aged 5 to 11. Photo by Marcelo Hernandez/Getty Images
COVID Vaccines for Kids Ages 5 to 11: Answers to Common Questions
BU infectious disease expert explains dosage and safety profile, and why immunizing elementary school–aged children is important
Some 28 million Americans still aren’t eligible for coronavirus vaccines—elementary school–aged kids between the ages of 5 and 11—but on Wednesday, Biden administration officials announced that’s poised to change in a matter of days.
The White House announced that it’s expected the Federal Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) will soon approve and recommend a scaled-down version of the Pfizer-BioNTech vaccine for pediatric use. Rather than rely on the mass inoculation sites that got shots into the arms of adults and kids aged 12 and up, officials say the rollout plan for vaccinating 5- to 11-year-olds will take place in 25,000 pediatric or primary care offices across the United States.
The FDA is expected to meet and discuss an emergency authorization of the Pfizer-BioNTech vaccine for 5- to 11-year-olds on Tuesday, October 26, opening the door for an FDA ruling and CDC recommendation to be in place by early November. With the school year well underway, and holiday travel just around the corner, the rollout of pediatric doses could come just in the nick of time to stave off more infections this winter.
To learn more about the scaled-down vaccine for kids and how it will impact the overall dynamics of the ongoing pandemic, The Brink reached out to Boston University infectious disease specialist Sabrina Assoumou, a BU School of Medicine assistant professor of medicine. She is also an attending physician at Boston Medical Center, BU’s primary teaching hospital and the city of Boston’s safety-net hospital.
Q&A
With Sabrina Assoumou
The Brink: How do the vaccines for 5- to 11-year-olds differ from vaccines for older kids (12 years and up) and adults?
Assoumou: The vaccine currently being evaluated by the FDA and CDC for 5- to 11-year-olds uses the same technology. The Pfizer vaccine is an mRNA vaccine. The main difference between the vaccine given to individuals older than 12 years and the one currently being evaluated for 5- to 11-year-olds is that a lower dose will be used for the younger children. One-third of the adult dose was shown to be safe and provide a robust immune response for 5- to 11-year-olds.
The Brink: Why will the vaccines be rolled out differently—through pediatric offices—instead of mass inoculation sites like we saw for adults?
Assoumou: The White House is shifting the approach for 5- to 11-year-olds, because there are data showing that parents often prefer to have their children vaccinated in locations that they are familiar with and with clinicians who are trusted messengers. We also have experience from prior vaccination efforts showing that vaccinating children in pediatric offices has been successful. In addition to pediatric offices, vaccines will also be available at hospitals, health centers, and some community sites.
The Brink: How much are children aged 5-11 currently contributing to the spread of COVID in the US? What effect will vaccinating this group have on life in America?
Assoumou: Recent data during the Delta wave showed that children can get infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and that they can transmit it. We also know that during a time when Delta is the dominant variant, we will need a high level of immunity in the community to decrease transmission. Given that children can transmit the virus and that there are 28 million children aged 5-11 in the US, it will be important to also vaccinate them so that we can decrease the level of transmission in the community.
The Brink: What can you tell parents about the safety aspect of the COVID vaccines for children?
Assoumou: The Pfizer vaccine is safe and provides a robust immune response for children 5 to 11 years of age. If parents have questions about the safety and efficacy of the vaccine, they should talk to their child’s healthcare professional to have their questions answered. In terms of real-world clinical data in children or young adults, a recent study published by the CDC showed that two doses of the Pfizer vaccine are highly effective in preventing COVID-19 hospitalization among persons aged 12-18 years. We are hoping to have real-world data for younger children once the vaccines are authorized for this age group, but we have information showing that the vaccines provide advantages for young adults.

Comments & Discussion
Boston University moderates comments to facilitate an informed, substantive, civil conversation. Abusive, profane, self-promotional, misleading, incoherent or off-topic comments will be rejected. Moderators are staffed during regular business hours (EST) and can only accept comments written in English. Statistics or facts must include a citation or a link to the citation.