Schaus Group Develops New Asymmetric Route To α-Amino Acid Esters
Chemical & Engineering News: Science & Technology (5/19/08) reports in a “Highlight” that Sha Lou and Scott E. Schaus have developed the first asymmetric catalytic version of the reaction.
It uses chiral biphenol catalysts to convert alkenyl boronates, secondary amines, and glyoxylates to chiral α-amino acid esters with good yields and high enantiomeric ratios (J. Am. Chem. Soc., DOI: 10.1021/ja8018934). The advance eliminates the need for stoichiometric chiral starting materials and opens the way to a broader range of products.
The work was done through the NIN-funded Boston University Chemical Methodology and Library Development Center
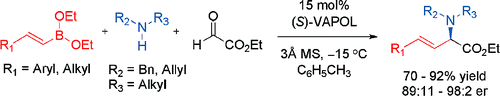
.