Teflon has nothing on the lotus plant; droplets of water that land on the surface of the plant’s leaf roll right off, sweeping away dirt particles as they go.
That extraordinary water repellence is known as superhydrophobicity, and scientists would love to harness it for such things as removing droplets from the surface of airplane wings before they freeze to reduce icing or improving the performance of steam turbines by keeping them dry.
“There are a lot of applications where people really want to get drops not just off surfaces, but off surfaces as fast as possible,” says James Bird, a College of Engineering assistant professor of mechanical engineering and head of ENG’s Interfacial Fluid Dynamics Laboratory.
Bird is studying how to minimize the contact time of a drop on a surface, and while he hasn’t bested nature, he may be coming close.
The lotus leaf and other superhydrophobic surfaces have two things going for them: a liquid-repellent surface chemistry—like a Teflon pan—and a texture that may appear smooth to the naked eye, but is actually roughened by microscopic pits, spikes, and pores. The microscopic texture traps air, so the drop is touching only a fraction of the solid surface.

ENG’s James Bird uses high-speed cameras to capture the bounce of a droplet. Photo by Jackie Ricciardi
When a raindrop-sized droplet lands on a superhydrophobic surface, it flattens out like a pancake and then rebounds, leaving the surface 12.4 milliseconds after it arrived.
Bird wanted to figure out how to reduce that contact time. What if the drop retracted in a different way, he wondered. If it splashed unevenly, the drop would theoretically leave the surface faster. “That was our thesis,” he says, “and, spoiler alert—we showed you can do that.”
He and colleagues from MIT found that by changing the surface on which a drop falls, they could change the hydrodynamics of the drop and the contact time. “Our goal was to have something that was manufacturable, more so than just an idea,” Bird says.
They used a laser to etch a rough, microscopic texture onto a silicon wafer, and then added ridges, which were smaller than the droplet, but big enough to see—about the size of a human hair. They captured the bounce using high-speed cameras.
Bird discovered that the drop bounced off the silicon wafer in 7.8 milliseconds, 37 percent faster than off the standard superhydrophobic surface. The explanation: when the drop spreads out into the pancake shape, the thickness varies over the hairlike ridges. That variation leads to variation in retraction speed, which causes the drop to split up and leave the surface more quickly than it would otherwise.
Next, they wanted to learn what would happen if they changed the surface microtexture and the material, from silicon to aluminum and copper. “We see the same dynamics,” says Bird, the lead author on a paper describing the results in the November 21, 2013, issue of Nature.
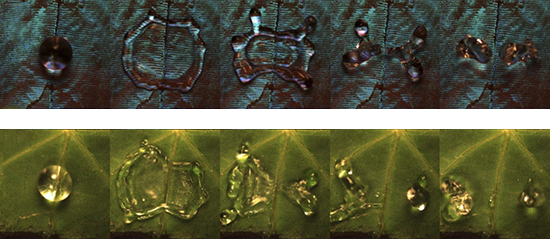
Bird and his team looked to nature for examples of superhydrophobic surfaces similar to the one they created, and found them in the wings of the Morpho butterfly and the leaves of the nasturtium plant. These scanning electron microscope images show droplets falling on a vein on a Morpho wing (top) and a vein on a nasturtium leaf (bottom). Courtesy of James Bird
Finally, they looked to nature for examples of superhydrophobic surfaces similar to the one they created, with both a microscopic texture and the larger ridges. They found those examples in the wings of the Morpho butterfly and the leaves of the nasturtium plant. Bird points to an image, taken by a scanning electron microscope, of the butterfly wing. “You have this roughness,” he says, “making it superhydrophobic, but you also have these veins—and the same for veins on the nasturtium leaf—that cause drops to come off the surface faster. So drops impacting the nasturtium leaf are shed faster than on the lotus leaf.”
Bird thinks the faster bounce rate may be caused by the way the veins are arranged. On the lotus leaf, the veins are just beneath the surface; on the butterfly wing and the nasturtium leaf, they’re on top. “So even though they both have veins that are about the same size, the position of those veins has the effect where one is going to lead to this drop breaking up faster, and the other is not,” he says.
As an engineer, Bird is more comfortable talking about the implications for airplanes and steam turbines than for butterflies and plant leaves. “We’re not doing as well as the butterfly wing,” he says. “But we’re approaching it.”













































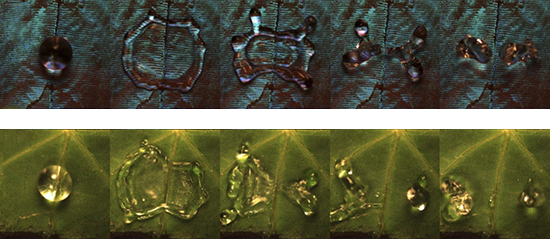
Related Stories
Six Quick Stats about Applicants to the Class of 2022
Record-breaking 64,470 applied, surge in those seeking early decision
Vinod Sarin Elected to National Academy of Inventors
BU materials scientist says nature inspires him with best ideas for new technologies
Fighting H1N1 with Tobacco Plants
Project earns Joseph von Fraunhofer Prize for ENG’s Sharon
Post Your Comment