Can We Find a Cure for Alzheimer’s Disease?
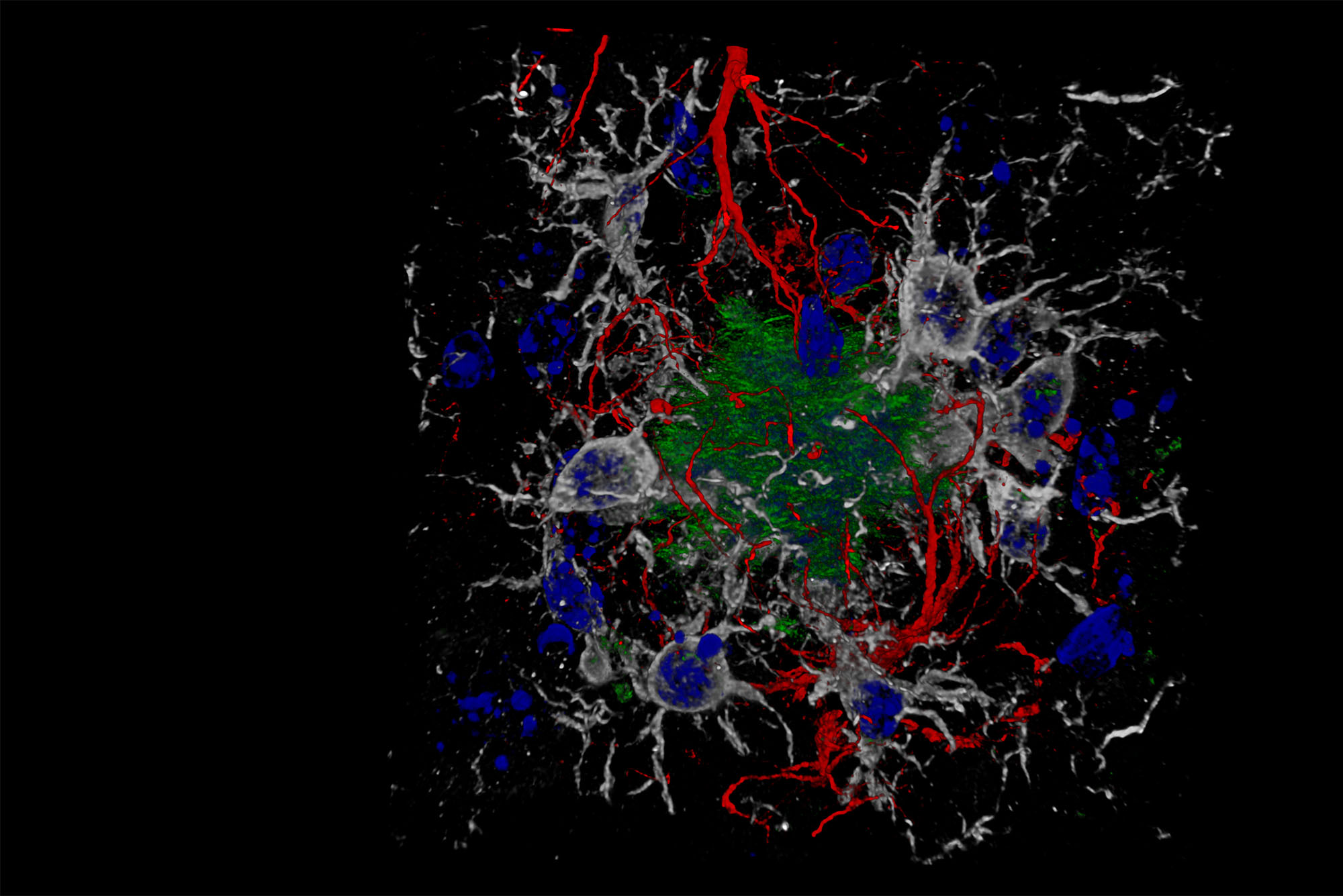
A composite image showing cells and beta-amyloid protein plaques—which disrupt cell function—in a brain with Alzheimer’s disease. BU researchers are studying how to better diagnose, prevent, and treat the disease. Image via Wikimedia Commons/Nicocapelo
Can We Find a Cure for Alzheimer’s Disease?
BU researchers are taking on this daunting question through a variety of approaches—and studying how to care for a growing population of people with the disease
Perhaps it starts with a quick memory lapse—a blank space where a loved one’s name should be, or a mysteriously empty key hook right before heading out. These are normal moments of forgetfulness that accompany aging. But then maybe this forgetting gets more concerning: long-cherished memories suddenly disappear and don’t come back easily (or at all), or behavioral changes cause unexpected hostility toward friends and family. By now, it’s clear: this is no longer simple, age-related memory loss; this is something more complex. Something such as Alzheimer’s disease.
Around the world, more than 55 million people have dementia, the majority with Alzheimer’s, a degenerative brain disorder that’s the result of damage to the brain’s nerve cells and causes memory loss, behavioral changes, confusion, and deterioration of language skills. In the United States, more than 6.7 million people over the age of 65 are living with Alzheimer’s, according to the Alzheimer’s Association, and the likelihood of developing the disease only goes up with age. And, while there are a number of therapeutic treatments available to people with the disease, there’s currently no cure.
Will there ever be?
That’s the question a number of researchers at Boston University are tackling, and with vastly different approaches. Among many others, there’s Ann McKee, a William Fairfield Warren Distinguished Professor and a Chobanian & Avedisain professor of neurology and pathology, who is searching for answers to Alzheimer’s at a genetic level; Wendy Qiu, a Chobanian & Avedisian School of Medicine professor of psychiatry who is exploring the causes of Alzheimer’s; and Ioannis Paschalidis, a College of Engineering Distinguished Professor of Engineering, who has developed an artificial intelligence–powered program to detect the disease.
The University is also home to the Alzheimer’s Disease Research Center, one of 33 such organizations in the United States funded by the National Institutes of Health to advance research on Alzheimer’s disease and related dementias. At the same time, other BU researchers are exploring ways to improve the quality of care for patients who currently have the disease, as well as build out resources for their families and caregivers.
Indeed, where some might see grim statistics and an uphill battle, dozens of geneticists, medical scientists, and other researchers at BU see opportunity—a chance to take on one of society’s greatest medical challenges. Here, The Brink explores three approaches, by three different researchers, aimed at improving treatment, diagnosis, and care for people with Alzheimer’s.
Genes: A Good Fit for a Cure
When Lindsay Farrer was a young medical geneticist, he happened to notice a family genealogy (also known as a pedigree) splayed across his advisor’s desk. It was 1984, and Farrer was finishing up his doctoral research on families with Huntington’s disease, a fatal inherited disorder that causes neurons to wither away.
He guessed the pedigree belonged to a Huntington’s patient. His advisor corrected him: Alzheimer’s disease.

Neither Farrer nor his advisor—nor anyone, really, for that matter—knew much about Alzheimer’s at the time. But they vowed to learn more. Now, close to 40 years later, many millions of dollars have been raised in the name of Alzheimer’s research, and Farrer is chief of the Biomedical Genetics section in BU’s Chobanian & Avedisian School of Medicine. In collaboration with other laboratories worldwide, Farrer’s group has localized genetic variants that are associated with a variety of rare and common disorders, including, notably, Alzheimer’s disease.
For the last 35 years, Farrer has focused his research on understanding and identifying genetic factors that make a person more at risk for, or more resilient to, developing the disease. In a seminal 2007 paper published in Nature Genetics, an international collaboration of researchers codirected by Farrer demonstrated that variants in the protein-encoding gene, SORL1, are associated with Alzheimer’s disease in multiple ethnic populations. The discovery, says Farrer, opens up a new potential target for Alzheimer’s researchers.
Because genes are inherited, “they can potentially impact Alzheimer’s disease mechanisms before any other environmental influences take root,” says Farrer, who is also a BU Distinguished Professor of Genetics and director of the University’s Molecular Genetics Core Facility. “We’re trying to figure out what are the genetic risk factors and protective factors, because they can serve both as potential diagnostic or predictive markers, as well as become potential therapeutic targets.”
We’re trying to figure out what are the genetic risk factors and protective factors, because they can serve both as potential diagnostic or predictive markers, as well as become potential therapeutic targets.
New drugs, including one approved by the US Food & Drug Administration in 2021, target a specific protein called beta-amyloid that’s believed to be especially toxic for the brain. According to the National Institutes of Health, scientists have found that “in the Alzheimer’s brain, abnormal levels of this naturally occurring protein clump together to form plaques that collect between neurons and disrupt cell function.” The goal of many pharmaceutical treatments so far is to interrupt the production of these harmful plaques in the brain, in a theory that’s known as the amyloid cascade hypothesis.
But Farrer and others believe that a better answer may be found somewhere earlier, and more foundational to the developmental process. Something like genes.
“We need to be looking at other targets that are more upstream, because by the time you’re tackling something [such as beta-amyloid] that’s that far down the cascade, it’s too late—brain cells have died or been injured, and they don’t recover. So, you want to get ahead of the game, you want to intervene before the cascade leading to the end stage pathology occurs,” he says.
Giving Voice to a Better Diagnosis
Trained in cognitive psychology, Rhoda Au approaches Alzheimer’s disease from a different angle, exploring ways to bring often crude neuropsychology testing tools up to speed for a digital age.
Au, a BU medical school professor of anatomy and neurobiology and one of the principal investigators of the Framingham Heart Study Brain Aging Program, is interested in moving away from traditional paper-and-pencil testing methods toward more sophisticated—and more natural—digital behavioral data collection.

Starting in 2005, she began digitally recording people’s voices as they answered standard psychological test questions as part of her work with the brain aging program. What Au later realized, inspired by the rise in digital voice assistants such as Apple’s Siri and Amazon’s Alexa, is that her recordings contained rich information about the participants—not just the content of their answers, but the quality of their voice and memory.
Au and her colleagues can analyze a person’s speech over time for changes that may indicate something cognitive—perhaps Alzheimer’s—is at play.
“Speaking is a cognitively complex task. And so, in that search for how to better measure people’s cognition, I realized an answer is embedded in those voice recordings. How so? Because, as you’re producing speech, you have to bring in a number of cognitive capabilities: you have to bring in your semantic memory, you have to be able to multitask and assemble a sentence, a quote, and then you have to bring together sentences that make sense into a coherent message. That’s all cognition, memory, attention, executive function; all of that comes into play,” she says.
Armed with her discovery, Au continues to push the field of Alzheimer’s research and diagnosis to use more innovative digital testing techniques that capture much richer, nuanced data.
For example, Au’s team is part of a new program, SpeechDx, launched by the Alzheimer’s Drug Discovery Foundation. It’s “a longitudinal study aimed at creating the largest repository of speech and voice data to help accelerate the detection, diagnosis, and monitoring of Alzheimer’s disease,” according to a trade magazine specializing in speech technology.
BU is one of several institutions collecting speech and other clinical brain-health data from more than 2,500 participants. All that data will be collated to create an enormous database that can be leveraged to spot trends and possible diagnostic markers—as a means for earlier Alzheimer’s detection overall.
Caring for an Aging Population
Aging, says Andrew Budson, “is simply the largest risk factor” for Alzheimer’s disease. And, with advances in preventive healthcare over the last several decades, people are generally living longer than ever.
“And so, because people are living longer, more and more people are developing Alzheimer’s disease as the number one late-life disorder that affects thinking and memory,” says Budson, a BU medical school neurology professor and associate director of BU’s Alzheimer’s Disease Research Center. For these people, Budson says, recently approved pharmaceutical treatments, including aducanumab and lecanemab—while not cures for the disease—can be a game changer.

“The world of Alzheimer’s disease is about to undergo an incredibly rapid, dramatic change. Because now, for the first time, instead of having medications that can just help with the symptoms a little bit, we have medications that can actually slow down the disease process,” he says, giving people with Alzheimer’s more time before severe dementia symptoms appear.
Still, Budson points out that these therapeutic treatments aren’t yet preventing anyone from developing Alzheimer’s, nor curing the disease altogether. The current best-case scenario is one in which “maybe twice as many people are living in their communities with mild memory problems,” he says—a reality he’s helping to prepare for.
He and his colleagues have, over the years, created a number of memory aids and strategies to help people with Alzheimer’s maintain a better grasp on when, how, and, indeed, whether, an event in their memory occurred—and better strategies for encoding new memories.
In someone with Alzheimer’s disease, their episodic memory is usually the first to be affected, Budson says. This is the form of memory that includes information about past events and experiences, such as where you parked your car. This is why a lot of people who are diagnosed with Alzheimer’s at first complain of short-term memory loss, Budson says. However, a patient’s procedural memory—seemingly unconscious information about how to complete a task—generally remains well preserved. This distinction led Budson to an important insight about how to help Alzheimer’s patients encode new memories, which is one of the topics of his recent book, Six Steps to Managing Alzheimer’s Disease and Dementia: A Guide for Families (Oxford University Press, 2021).
More and more people are developing Alzheimer’s disease as the number one late-life disorder that affects thinking and memory
Consider a parent who recently moved into a nursing home, for example. Rather than describing to them how to get to the cafeteria (“take a left, then the second right down the hall”), Budson suggests walking them there in person.
“If you give the person verbal information to remember, they have to use their episodic memory to remember it,” he says. “But if you walk them there, then you’re basically teaching their feet what to do,” a way of tapping into their procedural memory.
Even with these insights, it will take all of us to care for a growing population of aging people with, inevitably, some form of memory loss. And Budson emphasizes the importance of community care.
“This is research that I would say maybe isn’t as sexy as developing a pill or something like that,” Budson says wryly. “Disease-modifying medications slow down the progression but don’t stop the progression, and they don’t cure the disease. So, we have to think about what this is actually going to look like, as a society. And we need to consider what we’re going to do with all these people with mild memory problems: how do we help them to be more functional within their communities?”
For the community of BU researchers studying this vexing disease, the future holds promise, even if the journey has been rocky.
“You toil away at something for so long, carving out a path, until at some point, the stars align and suddenly you’re not on this journey alone anymore,” says Au. It’s a sentiment echoed by her BU colleagues, as well.
This Series
Also in
Aging Reimagined
-
February 21, 2024
The Secrets of Living to 100
-
February 21, 2024
Rethinking Our Idea of “Old Age”
-
February 21, 2024
The Ingredients of Unequal Aging: Housing, Income, and Health


Comments & Discussion
Boston University moderates comments to facilitate an informed, substantive, civil conversation. Abusive, profane, self-promotional, misleading, incoherent or off-topic comments will be rejected. Moderators are staffed during regular business hours (EST) and can only accept comments written in English. Statistics or facts must include a citation or a link to the citation.