Comprehensive Risk-Based Laboratory Inspection Program
Prepared by: Environmental Health & Safety
1. Introduction
Academic research and teaching laboratories at Boston University (BU) and Boston Medical Center (BMC) use a variety of hazardous materials (e.g., chemical, biological, radiological), perform procedures with potential risks, use laboratory equipment (e.g., centrifuges, x-rays, lasers) that pose potential hazards, or pose risks of fire and life safety hazards (e.g., via flammable materials, electrical/electronic equipment).
These materials, activities, and equipment are regulated by local, state, and federal agencies and have established industry standards as well as best safe practices. Many of these regulations require oversight provisions that mandate:
- Submission of application or permits for review and approval (e.g., biological materials use);
- Verification post-approval (e.g., environmental health and safety surveillance) and periodic reviews, audits, and inspections to ensure adherence to regulations, standards, and policies;
- Maintenance of detailed inventories of hazardous materials (e.g., chemical or radiological), animals, and controlled substances;
- Completion of required trainings for individuals engaged in research activities;
- Unannounced visits by regulatory agencies and;
- Reporting of accidents and incidents.
2. Purpose and Scope
2.1. Environmental Health & Safety (EHS) is committed to maintaining a safe laboratory environment for researchers, staff, students and visitors at BU and BMC. The objectives of the laboratory safety inspection program are:
- Monitor and verify the laboratory’s continued adherence to appropriate regulations, standards, and policies;
- Identify potential hazards in the workplace and provide recommendations on how to safely work with, store, and dispose of hazardous wastes;
- Confirm that corrections are made to address hazards in the work place;
- Explain and review observations found during inspections with the lab’s Principal Investigator (PI), LSC or lab representative and provide recommendations to address the findings;
- Work with the laboratory and assist to resolve inspection findings;
- Conduct inspections in a professional manner.
A comprehensive inspection is performed by the designated Safety Specialist from EHS for each lab at least annually. All inspections are conducted with the Principal Investigator (PI), Laboratory Safety Coordinator (LSC) or a designee appointed by the PI. The Safety Specialist will assist the PI and researchers in staying informed of current laboratory safety requirements and identify any deficiency and how it is addressed. The following areas are covered during the inspection. Other areas pertaining to safety will also be covered.
- Laboratory facility and support areas
- Personal protective equipment (PPE)
- Personnel required trainings
- Safety equipment and primary barriers (e.g. chemical hoods and Biological Safety Cabinets)
- Laboratory safety practices and procedures
- Hazardous material storage
- Hazardous waste disposal
- Disinfection and decontamination
- Registrations, approvals, SOPs required by BU safety committees
At the end of each inspection, the Safety Specialist will review the outcome of the inspection, review each finding, explain how they can be corrected, and the overall date for completing the corrective actions. The inspections results are entered into BioRaft® and the PI or LSC could enter updates as findings are corrected. The Safety Specialist will immediately inform the PI, LSC or designee of any hazards found that could be immediately dangerous to life and health which could require laboratory work to be suspended until further assessment or correction is made.
3. References
3.1. Regulations
3.1.1. 29 CFR 1910.1450 – Occupational Exposure to Hazardous Chemicals In Laboratories
3.1.2. 29 CFR 1910.1030 – Occupational Exposure to Bloodborne Pathogens
3.1.3. Biosafety in Microbiological and Biomedical Laboratories, 5th Edition
3.1.4. NIH Guideline for Research Involving Recombinant or Synthetic Nucleic Acid Molecules
3.1.5. Boston Fire Prevention Code
3.1.6. NFPA 101 Life Safety Code
3.1.7. NFPA 45 Laboratories Using Chemicals
3.1.8. City of Boston Laboratory Registration Ordinance
3.1.9. Boston Public Health Commission Laboratory Regulation
3.1.8. 105 CMR 480.000 Minimum Requirements for the Management of Medical or Biological Waste (State Sanitary Code Chapter VIII)
3.1.9. Boston Public Health Commission Recombinant DNA Technology: Use Regulation
3.1.10. Boston Public Health Commission Disease Surveillance and Reporting Regulation
3.1.11. 105 CMR 700.000: Implementation of M.G.L. c.94C (controlled substances)
3.1.12. 42 CFR Part 73 Possession, 7 CFR Part 331 and 9 CFR Part 121, Use and Transfer of Select Agents and Toxins
3.1.13. 49 CFR DOT Hazardous Materials Transportation
3.1.14. IATA Dangerous Transportation
3.2 BU Policies
3.2.1. Chemical Hygiene Plan
3.2.2. Biosafety Manual
3.2.3. Institutional Animal Care and Use Committee
3.2.4. Institutional Biosafety Committee
3.2.5. Laboratory Safety Committees
3.2.6. Radiation Safety Committee
3.2.7. Controlled Substance Program
3.2.8. Integrated Pest Control
3.3 Other SOP
3.3.1. Biological Use Authorization Review by the Institutional Biosafety Committee (IBC)
3.3.2. Biological Use Authorization Site Assessment by EHSEnvironmental Health & Safety
3.4 Supplementary Documents
3.4.1. IBC Approval Summary Form
3.4.2. Lab Inspection Deficiency Form
3.4.3. BUA Site Assessment Form
3.4.4. PI Pre Inspection Report
3.4.5. Chemical Inventory Report
4. Definitions
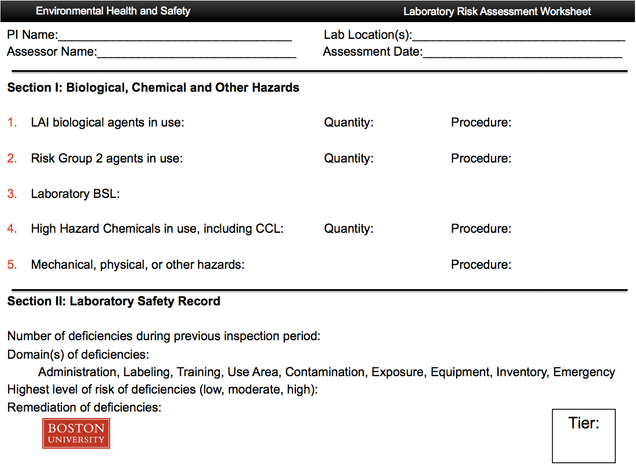
4.1 Tier Levels: Laboratory inspections are integral part of the risk assessment and management process. The frequency of the inspections is based on the hazardous materials present in the laboratory. A Tier-based inspections system is established for assuring the appropriate management and oversight of laboratories.
Tier 1:
Laboratories under the Tier-1 category generally do not work with chemicals that are classified as Highly Hazardous Chemicals (HHC) under BU Policy. Learn more about BU’s HHC policy
Agents used in these labs are not known to cause disease to immunocompetent individuals. This includes agents under Risk Group 1 (RG-1) or Biological Safety Level 1 (BSL1) as categorized by the National Institute of Health (NIH) and the Centers for Diseases Control and Prevention (CDC). A comprehensive inspection is performed on a Tier-1 laboratory annually.
Tier 2:
Laboratories under the Tier-2 category routinely use chemicals included in the BU HHC program. These labs also work with Risk Group 2 (RG-2) or Biological Safety Level 2 (BSL2) as characterized by NIH and CDC. Labs in this category also work with agents listed by the Laboratory Acquired Infection (LAI) Advisory Subcommittee of the IBC. Learn more about LAIs
Laboratories that use or conduct the following are also under the Tier-2 category:
- Use of controlled substances.
- Routinely employ processes or procedures that are determined by EHS to constitute a potentially significant hazard, including mechanical or physical hazards.
Laboratories under the Tier-2 category are inspected two times annually. The laboratory Tier designation is routinely reviewed and adjusted based on changes in materials used or processes performed in the laboratory. EHS works with the PI, LSC, or laboratory designee to ensure that the inspections are performed in a collaborative manner and to assist the lab achieve or maintain compliance.
Considerations for Relaxing Tier 2 Inspections to Tier 1 :
Laboratories classified under Tier 2 – based inspections may be considered as Tier 1 on a case-by-case basis when the lab meets the following criteria:
- Laboratories with a single HHC chemical may be considered under Tier 1-based inspection when it meets the following conditions:
- Laboratory only uses a single HHC – excluding hydrofluoric acid, perchloric acid, osmium tetroxide, MPTP, highly pyrophoric materials (e.g., tert-butyl lithium, diethyl zinc, or silane).
- Use of the HHC is limited (e.g., usage 2-4 times a year).
- The laboratory has adopted the current HHC SOP, maintains annual training records, and has all SOP required safety materials.
- The laboratory shows that it is consistently compliant with no findings.
- All related safety trainings are current.
- The laboratory does not use or store any other materials considered as Tier 2 under this policy.
- Laboratories using and storing only human sample materials including cells, cell lines, human tissue, human bodily fluids including blood, plasma, serum, and others may be considered under Tier 1 – based inspection when it meets the following conditions:
- The laboratory shows that it is consistently compliant with no findings.
- All related safety trainings are current.
- The laboratory does not use or store any other materials considered as Tier 2 under this .
- Laboratories storing and using Isoflurane only may be considered under Tier 1 – based inspection when it meets the following conditions:
- The laboratory shows that it is consistently compliant with no findings.
- All related safety trainings are curtrent.
- The laboratory does not use or store any other controlled substance and materials considered as Tier 2 under this .
- Laboratories with a single HHC chemical may be considered under Tier 1-based inspection when it meets the following conditions:
Revocation of Tier 2 Inspection Relaxation: Relaxation of Tier 2 inspections to Tier 1 will be revoked when the laboratory fails to meet any of the above stated conditions.
The BSL3 and BSL4 laboratories are designed as high and maximum containment laboratories and require more frequent inspections.
Follow-up Inspections:
EHS will assist laboratories and provide recommendations to resolve inspection findings. There are situations when EHS will work more closely with the laboratory to address corrective actions. In this instance, follow-up inspections may be conducted to ensure that corrective actions are successfully resolved.
Biological Use Authorization (BUA) Inspections
The Biosafety Officer (BSO) performs a BUA inspection and review of laboratories as part of the protocol approvals by the IBC. New protocols and protocols that are due for 3-year renewals are submitted to the BSO by the IBC office for review. The BSO meets with the PI to review the laboratory facility, procedures, and PPE to ensure that the facility is appropriate for the proposed work and meets the standards for the intended agents to be used.
Laboratory Self-Inspections:
Laboratories conduct voluntary self-inspections , to check the status of their laboratory space, equipment, process, and identify unsafe conditions. In some cases, EHS may request a laboratory to conduct self-inspections. The self-inspection focuses on health and safety issues and verification that the safeguards that are implemented are in place and followed. Upon completion, self-inspections results are entered into BioRaft.
4.2. Oversight Committees – Committees provide oversight for safety and compliance. Committees include; the Institutional Biosafety Committee (IBC), the Laboratory Safety Committee (LSC), the Intuitional Animal Care and Use Committee (IACUC), the Radiation Safety Committee (RSC), and the Institutional Review Board (IRB)
4.3. Principal Investigator (PI) – A faculty member or person with equivalent position that has overall responsibility for the safe operation of the laboratory facility and its personnel.
4.4. Laboratory Safety Coordinator (LSC) – A person appointed by the PI to supervise and be in charge of the daily laboratory operations, to ensure safety and compliance with safety, and to serve as a liaison with EHS
5. Procedures and Instructions
5.1. Frequency – Several oversight agencies have requirements that inspections be performed routinely and at least annually. EHS conducts a comprehensive inspection of laboratories and their support areas. The inspection covers major categories including biological safety, chemical safety, fire and life safety, and controlled substances. Laboratories at BU and BMC are inspected at least annually.
5.2. Ownership – EHS Safety Specialists are assigned to specific schools, departments, and/or principal investigators (PIs). These assignments encompass PIs and their laboratories that are actively conducting research or running teaching laboratories.
5.3. Scheduling Inspections – EHS will arrange a date and time with the laboratory to conduct the inspections. At the start of each inspection, the Safety Specialist informs the PI, LSC, or laboratory designee on the scope of the inspection. As needed, information may be sent to the laboratory prior to the inspection and would be covered during the inspection. Safety Specialist are expected to build a working relationship with the laboratories and assist them identify and address risks.
5.4. Pre-Inspection Preparation – To prepare for inspections, Safety Specialist review information on the laboratory to be inspected in BioRaft ®. This information includes but not limited to: last inspection report and status of any finding not closed; chemical inventory; status of current IBC protocols; and personnel trainings.
5.5. Inspection Process – The inspection starts with an opening meeting with the PI or their designee. The inspector will review the current status of the lab based on the information reviewed in the preparation prior to the inspection as well any information sent to the lab prior to the inspection. Any update or change provided by the lab will be noted and as needed, updated in BioRaft ®. Physical walkthrough of the lab and support spaces will be performed with the PI, LSC, or lab designee. The Safety Specialist will review all findings observed and recommendations at the conclusion of the inspection.
5.6. Reporting – Upon completion of each inspection, the Safety Specialist will issue an inspection report to the PI, LSC, or lab designee through BioRaft ®. The report outlines all the findings, including recommendations. The lab is required to provide a response outlining the actions taken to address each finding in BioRaft ®. EHS reviews the status of lab findings to ensure that they have been properly closed out by the lab.
EHS will also report any relevant changes in personnel or research status to the appropriate committees for follow-up (e.g., IBC, LSC, etc.). Depending on the nature of the findings, EHS may perform a follow up inspection of the laboratory to confirm that corrective actions have been resolved, or follow up on specific issues at the time of the next scheduled meeting with the laboratory safety coordinator.
6. Roles & Responsibilities
6.1. Environmental Health & Safety (EHS) Research Safety (RS) staff
6.1.1. Conduct comprehensive safety inspections of research facilities per this protocol.
6.1.2. Provide recommendations and assist Principal Investigators, Laboratory Safety Coordinators and other responsible parties in addressing and correcting findings identified during the inspection.
6.1.3. Inform laboratories of potentially hazardous situations and assist in mitigating the hazard.
6.1.4. Provide a laboratory inspection summary reports to appropriate committees including observed unsafe practices or continued non-compliance.
6.1.5. Report laboratories that are required to submit registrations or amendments to the IBC.
6.2 The Principal Investigator
6.2.1. Ensure their laboratories are safe and in compliance with Federal, State, and local safety requirements.
6.2.2. Ensure that findings identified during the inspection are resolved in a timely manner.
6.2.3. Provide the appropriate information regarding the laboratory in Research Information Management System (RIMS).
6.2.4 Appoint a Laboratory Safety Coordinator (LSC) for the laboratory. The LSC will coordinate with EHS and assist to provide information, answer questions, and address issues before, during, and after the inspection visit.
6.2.5. Report to EHS and any relevant oversight committees when corrective actions have been implemented.
6.2.6. Ensure that all members of the lab have completed their required training requirements.
6.3 Oversight Committees
6.3.1. Provide oversight of programs in the Committee’s technical area (i.e., biosafety, chemical safety, etc.).
6.3.2. Review inspection reports of laboratories that continue to be in noncompliance with applicable regulations and BU policies and recommend disciplinary actions as necessary.
6.4 Laboratory Safety Coordinator
6.4.1. As designated by the PI, be available to answer questions and address issues before, during, and after the inspection visit.
6.4.2 Ensure that corrective actions are implemented in a timely manner.
6.4.3 Communicate with the PI and other laboratory researchers about the findings of the inspection and any corrective actions that must be implemented.
7. Special Requirements: Deficiencies and Follow-up
Once a deficiency is identified an assessment will be made. Deficiencies may be categorized as “high risk,” “moderate risk,” or “low risk,” depending on several variables. An example of this would be improper PPE; while this may be low or moderate risk while working with non-high-hazard chemicals, it also has the potential to be an immediate danger to life and health (IDLH) if handling high-hazard chemicals (HHC).
Definitions
- Immediately Dangerous to Life and Health (IDLH) – A deficiency that may cause death or severe disability as found.
- High Risk – A deficiency that has the ability to cause death or severe injury if it is not remedied in a timely manner or is recurrent. High risk of environmental or building impact may also make a deficiency “high risk.”
- Moderate Risk – A deficiency that has the potential to cause harm or injury but is not severe or life threatening. The risk of moderate fines, building damage, environmental impact may also fall under “moderate risk.”
- Low Risk – A deficiency that will generally not cause harm to the user or environment, but may pose minor fines and/or do not comply with BU policy. Paperwork and minor labeling issues are examples of “low risk.”
- Frequency – If a single type of deficiency is noted repeatedly or frequently, it may result in an increase in the categorization of the risk. Observance of the same low risk deficiency in three consecutive inspections (including follow-up visits) will result in re-categorization of that deficiency as a moderate risk. Observance of the same moderate risk deficiency in two consecutive inspections (including follow-up visits) will result in re-categorization of that deficiency as a high risk. Observance of the same high-risk deficiency in two consecutive inspections (including follow-up visits) will result in re-categorization of that deficiency as IDLH.
Actions
Corrective actions will take place in accordance to the categories above. If any deficiency or hazard falls in the IDLH or high-risk category, immediate corrective actions must occur. Depending on the deficiency this may include resolving the issue at the time of the inspection, immediately notifying Facilities while remaining present at location, or stopping all work and/or evacuating until corrective actions can be taken. A report will also be forwarded to the Laboratory Safety Committee or the IBC, as appropriate, for review and follow-up.
If a deficiency falls into the moderate risk category, corrective actions must take place in a timely manner. Within 2 weeks of the initial inspection visit or at another reasonable time, EHS will conduct an unannounced follow-up visit to the laboratory to confirm that appropriate services are being provided and to assist the laboratory in addressing any open corrective items. The follow-up visit allows Research Safety staff to confirm that corrective actions are being implemented and consult with the Principal Investigator and/or Laboratory Safety Coordinator.
A deficiency that falls into the low risk category does not require immediate remediation. These deficiencies will be communicated during the inspection and noted in the inspection report. These deficiencies will be re-examined during the next inspection or follow-up visit.
8. Forms
See Appendices, below.
9. Training
See Laboratory Safety Training Program
10. Records Management
All records of inspections are saved in the BioRAFT
11. Program Revision History
This document as well as the inspection checklist will be reviewed at least annually by the research safety division.
12. Appendices
Appendix I: Risk assessment worksheet (tier assignment)
Read BU's full Laboratory Compliance Policy