BOTLab Research Projects
Our group develops diffuse optical technologies for clinical and translational applications, with a focus on cardiovascular disease, rheumatological disorders, cancer, kidney disease, and related conditions. Below is an overview of our current research projects.
Project 1: Speckle Contrast Optical Spectroscopy for Cuffless Blood Pressure
- Clinical focus: Hypertension, cardiovascular disease
- Core technology: Speckle Contrast Optical Spectroscopy (SCOS)
- Goal: Continuous, cuffless blood pressure monitoring
Speckle Contrast Optical Spectroscopy (SCOS) uses coherent laser illumination to quantify the volumetric flow of red blood cells within tissue. We are adapting SCOS to capture beat-to-beat changes in blood flow and volume across the cardiac cycle, enabling estimation of blood pressure without the use of an inflatable cuff.
Current work focuses on miniaturizing the instrumentation for unobtrusive, continuous monitoring and on rigorously validating accuracy through studies in healthy volunteers and patients with hypertension.
Collaborators: David Boas (BU BME), John Forman (BMC), Naomi Hamburg (BMC)
Funding: NIH NHLBI R01 R01HL177304
Why this matters: This work enables continuous, non-invasive blood pressure monitoring and may fundamentally change how hypertension is diagnosed and managed.

Project 2: Spatial Frequency Domain Imaging for Monitoring of Scleroderma
- Clinical focus: Autoimmune and rheumatological disease
- Core technology: Spatial Frequency Domain Imaging (SFDI)
- Goal: Quantitative, longitudinal assessment of skin involvement
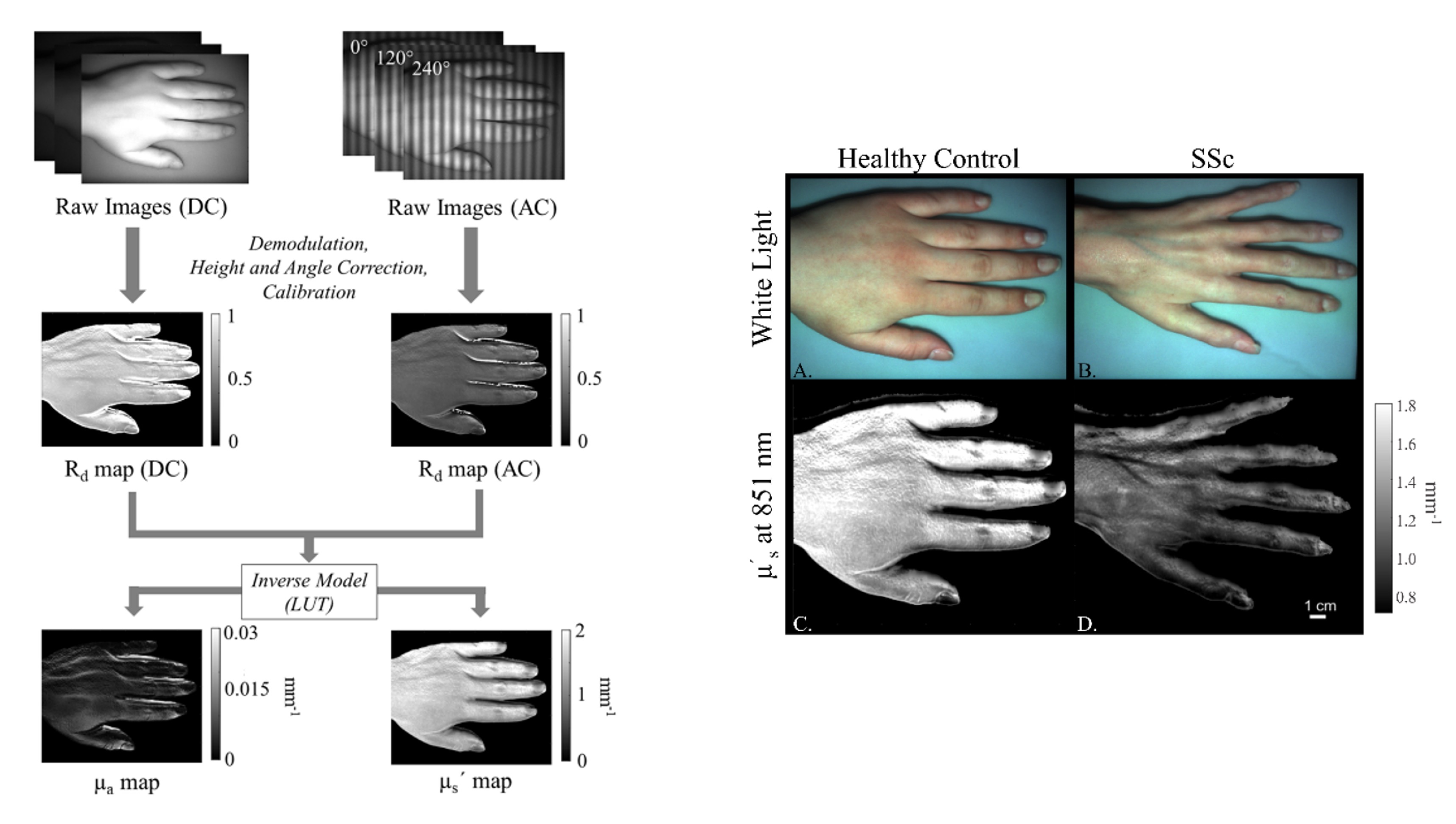
Spatial Frequency Domain Imaging (SFDI) uses projections of patterned light to quantitatively measure tissue optical absorption and scattering. We are developing SFDI to assess the extent of skin involvement in scleroderma, a chronic autoimmune disease with highly variable clinical presentation.
We have demonstrated that optical scattering measured with SFDI differentiates scleroderma patients from healthy controls, and we are now evaluating the ability of SFDI to longitudinally track disease progression and treatment response over time.
Collaborators: Andreea Bujor (BMC)
Funding: NIH NIBIB R01 EB037660, NIH NIAMS R01 RAR085317A
Why this matters: Quantitative optical biomarkers could improve objectivity in disease assessment and reduce reliance on subjective clinical scoring.
If you are interested in building your own SFDI system, visit our openSFDI project.
Project 3: SWIR Imaging and Spectroscopy for Tissue Water and Lipid Measurements
- Clinical focus: Kidney disease, hydration, metabolic health
- Core technology: Shortwave infrared (SWIR) imaging and spectroscopy
- Goal: Quantitative assessment of tissue water and lipid content
Recent advances in shortwave infrared (SWIR) detectors have enabled renewed interest in the SWIR spectral region (approximately 900–2000 nm). Reduced optical scattering at these wavelengths allows deeper penetration into biological tissue compared to visible and near-infrared light.
We are developing quantitative SWIR imaging and spectroscopy methods to measure tissue water and lipid content. These techniques have applications in kidney disease, sports medicine, consumer wearables, and other clinical and physiological monitoring contexts.
Collaborators: Vipul Chitalia (BMC)
Funding: NIH Trailblazer Award 1R21EB030197, NIH NIDDK R21DK132784, Air Force Research Labs
Why this matters: Direct optical access to tissue hydration and lipid composition could enable new biomarkers for volume status, metabolic health, and disease monitoring.

Project 4: Wearable Oximetry for Breast Cancer Treatment Monitoring
- Clinical focus: Breast cancer
- Core technology: Near-infrared wearable optical spectroscopy
- Goal: At-home monitoring of tumor hemodynamics during therapy
We are developing a near-infrared (NIR) wearable probe to monitor breast tumor hemodynamics during neoadjuvant (pre-surgical) chemotherapy. The probe features a hybrid flex-rigid design that conforms to natural breast geometry and a high-density optode array that enables spatial mapping of oxygenation changes in three dimensions.
Our current technology goals include developing a version of this device suitable for unsupervised, at-home use by patients during the course of therapy.
Funding: American Cancer Society
Why this matters: Continuous physiological monitoring during treatment could enable early identification of therapeutic response and personalized treatment adaptation.
