Wearable Windows into Breast Tumors
ENG’s Darren Roblyer is developing technology to help doctors choose best therapies
When biomedical engineer Darren Roblyer set up his Biomedical Optical Technologies Lab (BOTLab) at BU after arriving in 2012, he immediately started cold-calling doctors. He told them about a device he’d made, a new way of imaging tumors that could help cancer patients. It’s worth your time, he told them.
“I knew I wanted to get my device into the clinic,” says Roblyer, a College of Engineering assistant professor of biomedical engineering.
That device allows doctors to peer through the skin into breast cancer tumors and see their response to chemotherapy. Its readouts are instantaneous, and preliminary studies offer hope that the technology may someday provide doctors with information that currently eludes them: an immediate alert when drugs aren’t working.

Darren Roblyer’s wearable sensors conform to the breast and use an array of LEDs and optical sensors that can noninvasively monitor a breast cancer patient’s response to chemotherapy over time. Photo by Jackie Ricciardi
“It’s a window into the tumor. You can actually see what’s going on biologically without taking a biopsy,” says Catherine Klapperich, an ENG professor of biomedical engineering and of mechanical engineering and associate dean for research and technology development.
Breast cancer is typically diagnosed with a biopsy after an abnormal mammogram; doctors rarely do additional imaging or biopsies to track progress. Rather, they prescribe a treatment plan, and for the most part, stick with it. The plan varies depending on the genetic makeup of the tumor, the tissue structure, and the size. For some women, particularly those with large tumors that would be difficult to remove without taking the entire breast, doctors prescribe medicine to shrink the tumor first.
The medicine typically includes chemotherapy, which has a range of debilitating side effects. But most women respond extremely well to this therapy—five years later, 75 to 90 percent are cancer-free, according to Naomi Ko, a School of Medicine assistant professor of hematology and medical oncology and a breast oncologist at Boston Medical Center (BMC).
It’s the remaining 10 to 25 percent Roblyer hopes his device will help. These women don’t respond to the chemotherapy and spend months suffering its side effects without any benefit. Currently, says Ko, the best tool she has to determine if a patient is responding is to feel the tumor with her fingers and get a rough measure of its size using a ruler. “What you don’t want is the tumor to grow, meaning the chemo is not working,” she says. “That happens. I’ve had to send a patient emergently to the operating room for surgery.”
If clinical tests over the coming years confirm his early results, Roblyer hopes his device will provide doctors with better information more quickly, so they can change a patient’s treatment plan and avoid wasted time and emergency surgeries. “We’d like to prevent patients from undergoing months of ineffective treatment,” he says. “The idea is to use optical feedback to personalize and improve treatment for each patient.”
The flare
Roblyer got his first experience working with cancer patients as a Rice University graduate student in biomedical engineering, studying under Professor Rebecca Richards-Kortum. He had landed a spot in a Howard Hughes Medical Institute program intended to bring biomedical engineers closer to medical practice by putting them to work at nearby medical centers.
As part of the program, Roblyer worked at the Texas Medical Center, home of the MD Anderson Cancer Center. He dissected a cadaver to learn anatomy, followed physicians on their rounds, and attended surgeries. He also took a few cancer biology classes. “I became obsessed with cancer and medicine,” he says.
As a postdoctoral fellow, he joined the lab of Bruce Tromberg, a University of California, Irvine, professor and director of the Beckman Laser Institute and Medical Clinic. Tromberg was developing a new kind of imaging, called diffuse optical spectroscopic imaging (DOSI), for use in breast cancer.
The technology sends near-infrared light into tissue and measures what is reflected back. The technique can measure tissue several centimeters below the surface of the skin. Near-infrared spectroscopy is used in neuroscience research to detect brain activity near the surface of the brain. The light shines through the skin and skull and detects changes in blood flow that indicate brain activity.
In tumors, DOSI images reveal oxygenated blood flow, as well as fat and water content. These measures give a sense of the metabolic activity in the tumor, whether or not blood is flowing in different parts of it, and also if fluid is building up, potentially because of cell death. The technology captures changes instantaneously, so it can show real-time changes in response to the administration of a drug.

Biomedical engineer Darren Roblyer set up his Biomedical Optical Technologies Lab with the goal of making new devices that not only shine a light on cancer biology, but also help improve treatment for patients. Photo by Janice Checchio
By the time Roblyer joined Tromberg’s group, the team had used DOSI to measure several dozen breast cancer patients, all of them treated with chemotherapy to shrink the tumor prior to surgery. Roblyer analyzed the data, looking for signals that might be connected with a good outcome, meaning that the tumor had decreased in size, or a poor one, meaning that the chemotherapy was not effective.
He found one. In women whose tumors responded to chemotherapy, the DOSI images revealed a flare, a data spike that occurred early in treatment. The flare appeared within 24 hours of infusion with chemotherapy and represented an influx of oxygenated red blood cells into the tumor.
It isn’t clear yet exactly how the flare contributes to tumor shrinkage. Roblyer speculates that it may be a sign of inflammation, indicating that the tumor is being damaged by chemotherapy. “We’re still trying to figure out what’s going on at a molecular level,” he says.
Even without understanding the underlying biology, the information could still be valuable for prediction if it can be shown to occur in studies of larger numbers of patients. “The ability to measure the metabolism of these tumors gave us information about what was going to happen to those tumors months later. That’s powerful,” says Roblyer. “We’ve been chasing it ever since.”
What doors it will it open?
When Roblyer came to BU, his priority was to start those larger studies to validate his findings about the flare. He had reduced the size of the DOSI equipment needed to take measurements and wanted to get his new technology into a clinical trial. Earlier versions of the back end of the device, the part that produces the laser light, were the size of a refrigerator, but his current versions can be carried like a briefcase. “It gives us access to patients in new places, like the infusion suite at a cancer center,” he says.
His phone calls and emails to BMC oncologists led him to Ko, who agreed to run a clinical trial with him to test his technology. At this point, the tests involve collecting data from patients as they undergo chemotherapy prior to surgery. Roblyer and Ko will analyze that data to determine if his 2011 findings hold up in a larger group of women. If so, it will add to the evidence that the device could help doctors improve care for patients who don’t respond to chemo.

The spatial frequency domain imaging (SFDI) device takes measurements similar to those of the wearable sensors, to study how cancer responds to therapy in mice. Photo by Jackie Ricciardi
The probe used in the study with Ko is handheld and about the size of a brick. To get a full set of measures requires a few hours with the patient, and for this reason, it has been hard to convince patients to sign up. “A lot of these patients are already anxious about their first chemo, so to ask more of them is a lot,” she says.
But she does ask, and she is grateful when they agree. “We do it because we believe that this will further our understanding of breast cancer and how it responds to chemo,” Ko says. “I don’t know what doors it will open, but there’s great potential to learn.”
Until now, cancer researchers could see only snapshots of tumors from mammograms or examine tumor tissue after it’s removed from the body. “With this device we can see a living tumor reacting to the medicine being infused,” says Klapperich. “The technology is opening up the potential to answer new biological questions that couldn’t have been answered before.”
The ability to see dynamic changes, like the flare Roblyer found as a postdoc, is one thing. But to understand what’s happening inside the tumor to produce that flare and how that biological process might be linked to the shrinking of tumors requires more extensive digging, the kind of digging that isn’t possible to do in human patients.
So Roblyer also studies mouse models of breast cancer using the BOTLab’s imaging technologies. To launch this effort, fifth year graduate student Syeda Tabassum (ENG’18), who works in the lab, confirmed that it was possible to use a form of imaging called spatial frequency domain imaging, or SFDI, to image breast tumors in mice. Similar to DOSI, SFDI uses near infrared light and measures oxygen saturation, water, and fat content. SFDI also allows imaging of the tumors at multiple depths, so it is possible to create, for example, a map of oxygen saturation across the entire tumor.

A team of graduate students in the BOTLab, Fei Teng (ENG’18) (from left), Kavon Karrobi (ENG’19), and Syeda Tabassum (ENG’18), is continually developing smaller and more sensitive optical devices and testing them in the lab. Photo by Jackie Ricciardi
In new studies, graduate student Kavon Karrobi (ENG’19) is using SFDI in combination with another form of imaging, called multiphoton microscopy (MPM), which creates images of much higher resolution. Using MPM, Karrobi can image a cross section of the tumor and get a detailed look at the blood vessels, tumor cells, and structure of the tissue. It’s a bit like taking a slice of the tumor and inspecting it under a microscope, but without touching the tumor.
Combining these tools, Roblyer’s team can image the tumor throughout a course of treatment and see how it changes. The work is just beginning, but one observation so far is that tumors don’t change uniformly in response to treatment. “There are pockets within a tumor and we’re trying to understand how they relate to growth or resistance to therapy,” Karrobi says.
Wearable windows
Such heterogeneity within a tumor is common and well known, but Roblyer’s tools are allowing his team to visualize them in a completely new way. For instance, Karrobi plans to overlay detailed images of tumor vasculature onto maps of oxygenation. “One provides context for the other and could give us an idea of what is happening inside,” he says. “We’re still very much in the exploration phase.”
This research is helping Roblyer’s team learn more about how tumors behave and also what the signals they see with the imaging tools they are testing mean. The more Roblyer understands about the cellular and molecular processes his device detects, the more valuable those signals become. “We’re interested in what’s going on in a tumor over time,” he says. “We can image things other people can’t, so we’re learning a lot. It could go in many different directions.”
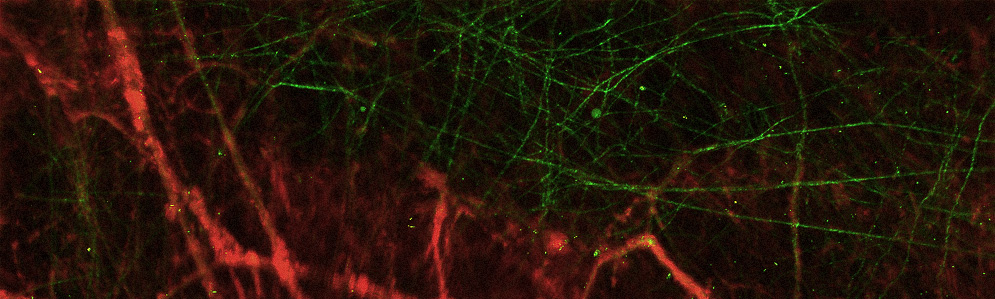
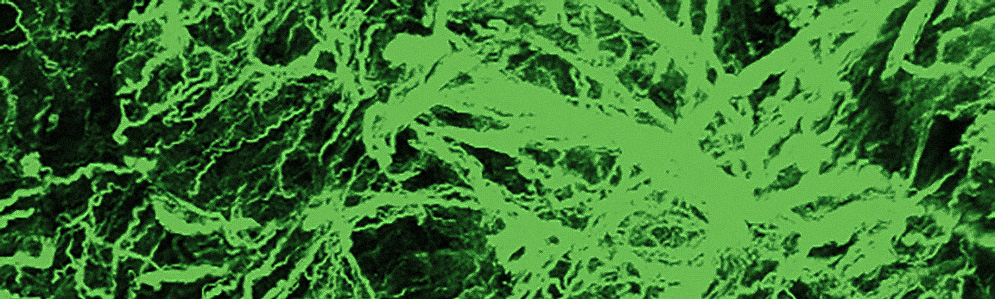
Roblyer’s team uses imaging tools in the lab that reveal the inner structure of a tumor, such as the shape and health of its blood vessels (left) or the tumor tissue’s collagen structure (right). This technology is too invasive to use on patients, but in laboratory studies of mice with breast tumors, they help Roblyer’s team understand more about how cancer changes as it responds to chemotherapy. Images courtesy of Darren Roblyer
Meanwhile, Roblyer’s lab continues to advance the DOSI technology. Last year, they created the first wearable DOSI device, improving upon the brick-sized probe. The wearable device is flat, star-shaped, and flexible so it can conform to the shape of the breast and be worn during a chemotherapy infusion, making it more convenient for patients.
To shrink the technology, however, Roblyer had to alter its function. His older probe collects absolute measures, but the new one detects only relative changes. It’s a bit like having a heart rate monitor that shows how much faster the heart beats during exercise, but cannot tell the starting or maximum heart rate.
Roblyer is also improving speed and resolution. For instance, he recently completed a second wearable design that has many more light sources and sensors, allowing it to probe more deeply and take a series of images at different depths throughout the tumor tissue, potentially revealing pockets of response or nonresponse to therapy in a patient’s tumor.
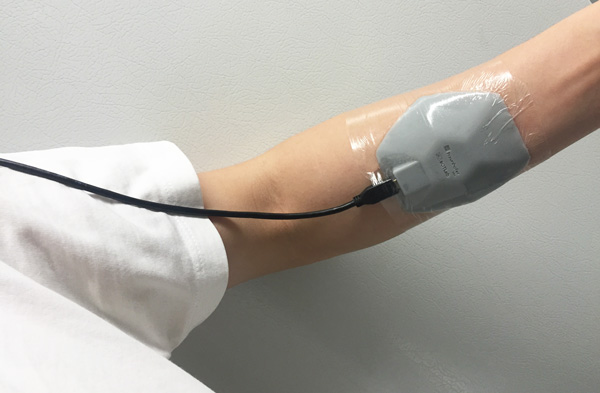
Before testing the wearable sensors on patients, Roblyer tests early designs of his devices on the wrists of healthy volunteers to see how well it measures blood flow and water and fat content. Photo courtesy of Darren Roblyer
He and his team of two postdoctoral fellows and seven graduate students have managed to create these new designs in less than two years. The seed money to create the first wearable probe came from the BU Center for Future Technologies in Cancer Care, directed by Klapperich. Roblyer also received a grant to support collaboration with the Fraunhofer Center for Manufacturing Innovation at BU, which helped create the flexible circuit board and skin-safe material in the wearable device. In addition, he has received funding from the American Cancer Society as well as a $4 million five-year grant from the US Department of Defense Breast Cancer Research Program.
He wants to take these newer devices to the clinic. He’s already at work on his next version: a wearable design that can collect absolute measures of oxygenated blood, fat, and lipids. All of his efforts must move forward in parallel, with his team learning from studies of patients and laboratory animals and adjusting their technology each step of the way.
“The most important thing,” says Roblyer, “is to figure out if this is the right technology and if it will help people.”
Elizabeth Dougherty can be reached at beth@writtenbyelizbethdougherty.com.
This Series
Also in
Breast Cancer Research
-
April 24, 2017
Zebrafish Cancer Genetics Illuminate Human Breast Cancers
-
April 24, 2017
Dioxins Point to Targets for Treating Breast Cancer
-
April 24, 2017
Too Many Black Women Die from Breast Cancer. Why?







Comments & Discussion
Boston University moderates comments to facilitate an informed, substantive, civil conversation. Abusive, profane, self-promotional, misleading, incoherent or off-topic comments will be rejected. Moderators are staffed during regular business hours (EST) and can only accept comments written in English. Statistics or facts must include a citation or a link to the citation.