2023 Symposium Abstracts
Keynote Speaker: Dr. Muthiah “Mano” Manoharan
 Senior VP, Alnylam Pharmaceuticals
Senior VP, Alnylam Pharmaceuticals
“Living in the World of Nucleic Acids Therapeutics: Challenges and Opportunities”
Synthetic small interfering RNAs (siRNAs) are potent inhibitors of gene expression. These molecules are perfect examples of biomimetic chemistry as synthetic siRNAs act through the natural RNA interference (RNAi) pathway. To deliver therapeutic siRNAs into human liver, we developed approaches that include chemical modification of the siRNAs and either lipid nanoparticle (LNP) formulation or multivalent N-acetylgalactosamine (GalNAc) conjugation, making possible intravenous and subcutaneous administration, respectively. The design of chemical modifications of siRNAs to enable favorable Argonaute2 (Ago2) recognition as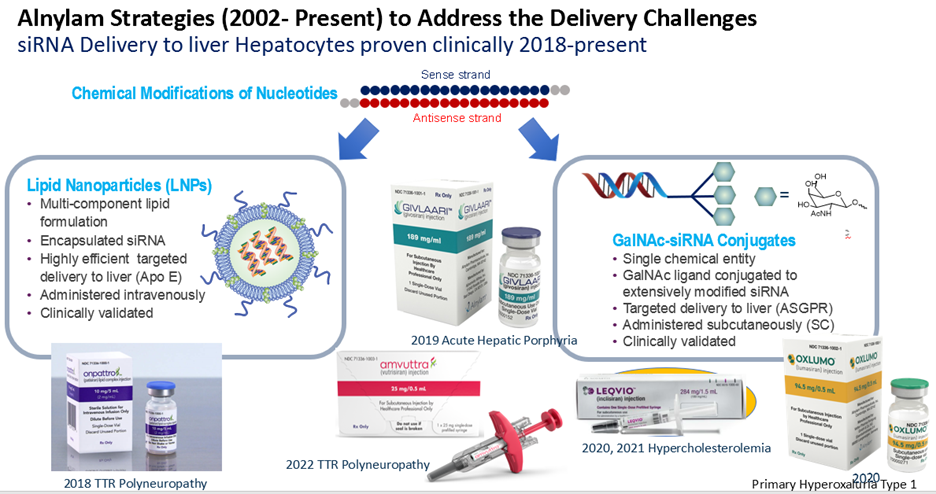 well as both delivery strategies rely on biomimetics. The LNP approach is based on the endogenous Apo-E ligand /LDL receptor process. Using these strategies five approved RNAi therapeutics have emerged from Alnylam. We have also used lipid conjugates for CNS delivery of therapeutic siRNAs.
well as both delivery strategies rely on biomimetics. The LNP approach is based on the endogenous Apo-E ligand /LDL receptor process. Using these strategies five approved RNAi therapeutics have emerged from Alnylam. We have also used lipid conjugates for CNS delivery of therapeutic siRNAs.
This presentation will cover the molecular basis of RNA therapeutics including the chemical modifications and motifs used in each RNA strand to ensure uptake into cells of the targeted tissue, Ago2 recognition, silencing efficiency, metabolic stability, and safety.
Faculty Speaker: Florian Douam, PhD
![]()
 Assistant Professor, Microbiology, NEIDL
Assistant Professor, Microbiology, NEIDL
“Modeling human immune responses to viruses in small animal models”
The human immune system displays structural and functional features that are not recapitulated in other animal species, including those routinely used in immunology research. This creates major challenges for comprehensively understanding the immunological mechanisms defining the effective control, or lack thereof, of viral infection in humans, and hinders our ability to develop innovative countermeasures with broad antiviral activities. Over the past decade, our laboratory and others have demonstrated that humanized mice, i.e., mice engrafted with human cells and/or tissues, represent powerful platforms to tackle such challenges through their ability to model human immune responses to infectious pathogens. In this presentation, I will discuss how recent advances in immunology, mouse genetics, engraftment protocols, and organoid development can foster the generation of a novel generation of humanized mouse models with the potential to revolutionize our understanding of human immune responses to viral infection. Specifically, I will introduce some of the humanized mouse models we have developed to investigate tissue-resident human immune responses against two clinically relevant types of viruses, namely arthropod-borne viruses and respiratory viruses.
Faculty Speaker: Michelle Teplensky, PhD
![]()
 Assistant Professor, Biomedical Engineering and Materials Science and Engineering
Assistant Professor, Biomedical Engineering and Materials Science and Engineering
“Harnessing Nanoscale Engineering to Program Immunity”
Vaccines can program a directed target-specific immune response and are attractive therapeutics because many cancers exhibit targetable tumor-specific mutations. Nevertheless, overall response rates to vaccination are low. This can be attributed to the inability to control the delivery and presentation of vaccine components to immune cells. Indeed, we have observed that this lack of nanoscale control directly inhibits the raised immune response. Moreover, even when vaccines activate immune cells, the tumor’s immunosuppressive environment prevents the response’s longevity. Therefore, we urgently need to develop strategies to harness vaccine design to effectively deliver immune activating and targeting components while preventing tumor immune evasion. In this work, we explored strategies to activate multi-faceted robust immunity by harnessing the nanoscale placement of immunostimulatory, immune targeting, and anti-immune evasive cues. We highlight the opportunity to uniquely program immunological interactions and positively impact vaccine efficacy across multiple tumor types and microenvironments through nanoscale arrangement. This emphasizes the critical role of nanoscale vaccine structural development to raise complex multi-faceted immunity against future diseases.
Faculty Speaker: Adriana Tomic, PhD
![]()
 Assistant Professor, Biomedical Engineering and Microbiology, NEIDL
Assistant Professor, Biomedical Engineering and Microbiology, NEIDL
“Creating Smarter Vaccines: Understanding Our Immune System with Artificial Intelligence”
With the potential to revolutionize healthcare, the systems immunology approach offers a comprehensive view of biological systems that is key to improving vaccine development. Harnessing the capabilities of high-throughput (omics) technologies, our overarching goal is to uncover the complex panorama of host-pathogen immune interplay. The challenge arising herein is the translation of comprehensive datasets into insightful knowledge that can deepen our understanding of immune responses and the progression of infectious diseases. We’ve tackled this challenge with a unique approach that combines high-level systems analysis with Artificial Intelligence (AI). Using this approach, we have uncovered detailed insights into how our body battles diseases and prevents them from causing harm. This methodology integrates multi-omics data, and computational modeling to predict population-wide immune response outcomes, identify protective correlates, and create a pathway for rational vaccine design. Our work has shed light on protective immunity signatures for diseases of paramount public health concern, such as influenza and SARS-CoV-2, underscoring the multifaceted nature of human protective immunity. To share these findings and help speed up global research efforts, we’ve made our AI platform freely available to all. Our approach, entailing systems-level, and computational integrative analysis, generates a wealth of insights into immune protection and memory mechanisms and drives innovative infectious disease prevention strategies.
Student Speaker: Joshua McGee, PhD Candidate
![]()
 PhD Candidate, Biomedical Engineering
PhD Candidate, Biomedical Engineering
“Overcoming the innate immunity of self-amplifying RNA to enable potent and enduring vaccines and therapies”
Self-amplifying RNA (saRNA) will revolutionize vaccines and in situ therapeutics by enabling protein expression for longer duration at lower doses. However, a major barrier to saRNA efficacy is the potent early interferon response triggered upon cellular entry, resulting in saRNA degradation and translational inhibition. Substitution of mRNA with modified nucleotides (modNTPs), such as N1-methylpseudouridine (N1mΨ), reduce the interferon response and enhance expression levels. Multiple attempts to use modNTPs in saRNA have been unsuccessful, leading to the conclusion that modNTPs are incompatible with saRNA, thus hindering further development. Here, contrary to the common dogma in the field, we identify multiple modNTPs that when incorporated into saRNA at 100% substitution confer immune evasion and enhance expression potency. Transfection efficiency enhances by roughly an order of magnitude in difficult to transfect cell types compared to unmodified saRNA, and interferon production reduces by >8 fold compared to unmodified saRNA in human peripheral blood mononuclear cells (PBMCs). Furthermore, we demonstrate expression of viral antigens in vitro and observe significant protection against lethal challenge with a mouse-adapted SARS-CoV-2 strain in vivo. A modified saRNA vaccine, at 100-fold lower dose than a modified mRNA vaccine, results in a statistically improved performance to unmodified saRNA and statistically equivalent performance to modified mRNA. This discovery considerably broadens the potential scope of self-amplifying RNA, enabling entry into previously impossible cell types, as well as the potential to apply saRNA technology to non-vaccine modalities such as cell therapy and protein replacement.