Toxic Tau
BU researcher links Alzheimer’s disease to brain cells’ faulty stress response

“I have lost myself,” the patient told her doctor.
It was 1901, and the woman, disoriented and delusional, had just been admitted to a psychiatric hospital in Frankfurt, Germany. Her memories were a jumble of missing and broken pieces. She could no longer summon up her own last name. Yet her symptoms couldn’t be dismissed as old age: at just 51, she was barely a decade older than the psychiatrist treating her, Alois Alzheimer.
When the woman died five years later, Alzheimer examined her brain cells under a microscope, identifying for the first time the physical degradation associated with the debilitating constellation of symptoms we now know as Alzheimer’s disease. He found two distinctive features: “plaques” between neurons and “tangles” inside them. Since then, most Alzheimer’s research and drug development has focused on the plaques, which are made up of pieces of protein called beta-amyloid. But in recent years, many researchers have turned their attention to the tangles, where accumulations of a protein called tau poison and kill brain cells.
Benjamin Wolozin, a professor of pharmacology and neurology at the Boston University School of Medicine (MED), is investigating what makes tau turn toxic. He and his team, including Tara Vanderweyde (MED’15) and current BU graduate student Daniel Apicco, both in the pharmacology program, have found that a malfunction in neurons’ stress response can trigger a feedback loop that causes tau to misfold and cluster. They have also found that by tamping down a protein called TIA1, they can stop this vicious cycle. They published their research in the May 17, 2016, issue of Cell Reports.
When a cell is injured, starved, or subjected to other kinds of stress, it declares a state of cellular emergency, explains Wolozin. The cell becomes like a city on lockdown: Special proteins called RNA-binding proteins flood out of the nucleus and round up all the RNA that the cell uses for ordinary business, isolating it in accumulations called “stress granules” and leaving behind only what the cell needs to repair itself. Typically, the lockdown is lifted after a few minutes, and the RNA is released from the stress granules so that the cell can get back to business as usual.
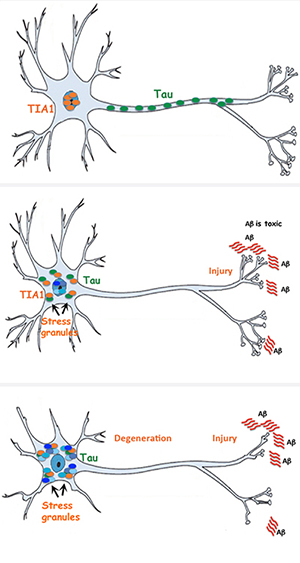
In Alzheimer’s, though, something goes wrong and the RNA stays locked up. Wolozin and his team wanted to find out why. The culprit, they discovered, is tau. Tau encourages cellular misbehavior by stimulating the formation of stress granules. Moreover, the team found that the stress granules cause tau to clump up and misfold, turning it toxic and creating the hallmark tangles that eventually kill brain cells.
“It’s well known that diabetes and cardiovascular disease are major risk factors for Alzheimer’s disease,” says Wolozin. He adds, however, that researchers have not understood how these conditions lead to brain degeneration. The new research helps illuminate one mechanism by which chronic cellular stress can foster the development of Alzheimer’s and other neurodegenerative diseases, like frontotemporal dementia and amyotrophic lateral sclerosis (ALS, or Lou Gehrig’s disease), which also seem to involve pathological stress granules.
The discovery also suggests that by holding back certain RNA-binding proteins—the “escorts” that usher RNA into stress granules—it might be possible to keep the stress granules from turning pathological and bringing on Alzheimer’s, says Wolozin. But the body uses some 800 different binding proteins: Where to start? Wolozin and his team picked the TIA1 protein, which tends to be the first molecule to build up at the site of a stress granule. Using brain cells from mice that don’t make normal amounts of TIA1 and tau, they showed that neurons with too much TIA1 made misfolded tau proteins and degenerated, while those with low concentrations of TIA1 resisted the accumulation of toxic tau.
Now, with support from the Edward N. and Della L. Thome Memorial Foundation, Wolozin is searching for a compound that could stave off Alzheimer’s by controlling tau and TIA1 levels in the brain. Using fast automated screening techniques, he plans to test some 150,000 different molecules over the next several months. Identifying a promising compound is just the beginning, though. “The brain is a privileged area,” says Wolozin. Many compounds are blocked entry by sophisticated cellular gatekeepers. In addition, to make it out of the lab and into the pharmacy, a drug must first be proven safe and effective in humans. (Wolozin is also the founder of Aquinnah Pharmaceuticals, which is currently developing drug therapies for ALS based on similar screening techniques. Aquinnah will co-develop with BU any promising compounds that come out of the screening campaign. In addition, Wolozin holds a patent, which Aquinnah is working with BU to license, on curbing the activity of the TIA1 gene as a way to treat Alzheimer’s.)
Alzheimer’s is a multifaceted disease, says Wolozin, with no single cause; neither TIA1 nor any other RNA-binding protein will be the complete answer to the many questions Alzheimer’s raises about how the brain works, in health and in sickness. But because these proteins have been little studied until now, Wolozin says, they have the potential to teach researchers a great deal very quickly. “Research is like surfing,” says Wolozin. “You look for a good wave to ride—and this is a good wave.”
Comments & Discussion
Boston University moderates comments to facilitate an informed, substantive, civil conversation. Abusive, profane, self-promotional, misleading, incoherent or off-topic comments will be rejected. Moderators are staffed during regular business hours (EST) and can only accept comments written in English. Statistics or facts must include a citation or a link to the citation.