Structure and Function
History
Interleukin 16 (IL-16), formerly known as lymphocyte chemoattractant factor or LCF, is a pro-inflammatory cytokine that is chemotactic for CD4+ T lymphocytes, monocytes, and eosinophils. In addition to inducing chemotaxis, IL-16 can upregulate IL-2 receptor and HLA-DR4 expression, inhibit T cell receptor (TcR)/CD3-dependent activation, and promote repression of HIV-1 transcription. IL-16 is a unique cytokine with no significant sequence homology to other well-characterized cytokines or chemokines.
Structure
IL-16 was originally identified as a homotetramer consisting of individual 14 kDa monomers of 130 amino acids (aa) each. IL-16 is synthesized as a precursor molecule (pro-IL-16) of approximately 68 kDa and 631 aa lacking a signal peptide. The gene for IL-16 maps to chromosome 15 in the human and chromosome 7 in the mouse. The sequence and overall structure of IL-16 is conserved across species. Both human and mouse IL-16 genes comprise seven exons and six introns. Human and mouse IL-16 homologs display conservation of both structure and function, particularly in the C-terminal region (82.1% similarity). The human pro-IL-16 sequence displays >90% similarity to various non-human primates.
Recombinant pro-IL-16 polypeptides are specifically cleaved in CD8+ cell lysates suggesting that the actual secreted form of IL-16 may be smaller than the originally published 130 aa form. One potential protease cleavage site within the C-terminal region of IL-16 occurs following an Asp residue implicating the involvement of a caspase family member. In CD8+ T cells, active caspase-3 cleaves pro-IL-16 producing a biologically active, secreted form of IL-16 (i.e., representing 121 C-terminal aa residues of pro-IL-16). The mechanism of release or secretion of IL-16, however, is currently unknown but does not appear to correlate with apoptosis.
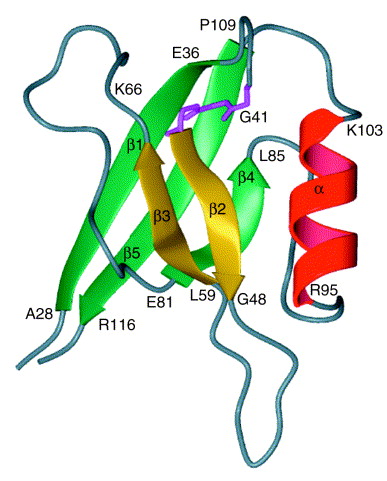
The aa sequence of mature, secreted IL-16 contains a PDZ motif. A PDZ motif consists of an approximately 90 aa residue unit, which is often repeated in a protein. They are typically intracellular protein modules involved in clustering ion channels, receptors, and other membrane proteins, connecting them to signaling complexes. A conserved GLGF (Gly-Leu-Gly-Phe) aa sequence characterizes PDZ motifs and functions in mediating binding of defined peptide sequences. The IL-16 PDZ motif does contain a “GLGF cleft”, however, it is much smaller than those of other PDZ-containing proteins and is blocked by a tryptophan side chain in its center. Blockage of the peptide-binding site prevents IL-16 from displaying expected peptide-binding properties of PDZ-like domains. The functional significance of the IL-16 PDZ motif remains to be identified.
How it is signaling
CD4 serves as a signal-transducing receptor for IL-16. Expression of CD4 is required for mediating IL-16 functions. Interaction between IL-16 and CD4 can specifically initiate an increase in intracytoplasmic calcium and inositol trisphosphate, activation of p56lck, and translocation of protein kinase C from the cytosol to the cell membrane. IL-16-induced functions can be inhibited by a monomeric Fab of an anti-CD4 (OKT4) monoclonal antibody. The region of CD4 that binds IL-16 has been identified within the D4 domain, overlapping structure involved in CD4 dimer formation. Studies with point mutations or deletions in the C- and N-terminal residues of IL-16 demonstrate that sequences within both the C- and N-terminal domains of IL-16 are required for interaction with CD4.
A synthetic peptide representing the 16 C-terminal aa residues of IL-16, Arg106 – Ser121, can inhibit IL-16 chemoattractant activity of CD4+ cells. This peptide also competes for binding of the OKT4 anti-CD4 monoclonal antibody suggesting that it is a competitive inhibitor for CD4 receptor binding. The minimal peptide sequence, RRKS (Arg106 – Ser109), can mediate the inhibition of IL-16 chemoattractant activity. Specifically, the critical residue in native IL-16 responsible for inducing CD4+ cell migration by binding or activation of the IL-16 receptor appears to be Arg107. In support of this observation, Arg107 is conserved in the IL-16 sequence of mouse and various primate species.

Bioactivity (posted on R&D)
Sources of IL-16 include epithelial cells, mast cells, lymphocytes, macrophages, synovial fibroblasts, and eosinophils. IL-16 mRNA is constitutively expressed in both CD4+ and CD8+ cells, however, synthesis is induced in T lymphocytes upon exposure to antigen or mitogen. IL-16 may also be secreted by activated CD8+ cells in response to histamine or serotonin. IL-16 expression has been linked to inflammation processes in asthma, rheumatoid arthritis, systemic lupus erythematosus, colitis, atopic dermatitis, and multiple sclerosis. For example, the expression of IL-16 directly correlates with the number of infiltrating CD4+ T cells in asthmatic epithelium. IL-16 has also been shown to play a role as a suppressive factor for HIV-1. Recombinant IL-16 (C-terminal 130 aa) represses HIV-1 replication in peripheral blood mononuclear cells. Human CD4+ cells transfected with IL-16 cDNA are resistant to HIV-1 infection, not at the level of viral entry or reverse transcription, but at the level of expression of mRNA. Apparently, secretion of IL-16 is required for HIV inhibitory activity since cells expressing either the C-terminal 130 aa or the C-terminal 100 aa without signal peptides are poor secretors of IL-16 and exhibit little, if any, resistance to HIV. Although both IL-16 and HIV-1 use CD4 as a receptor, studies have shown that they do not share a common binding site. The mechanism of HIV suppression by IL-16, therefore, does not involve steric inhibition of viral binding to CD4 as is the case with several CC chemokines (i.e., RANTES, MIP-1 alpha, and MIP-1 beta). Rather, IL-16 promotes the inhibition of HIV-1 promoter activity.
To conclude:
The use of IL-16 or IL-16 antagonists may have potential therapeutic value in various pathological immune responses. In HIV-1 infection, IL-16 may be used to promote CD4+ T cell immune reconstitution and repress HIV-1 replication. Analysis of serum samples from HIV-1-infected individuals demonstrate consistent or increased IL-16 levels during the asymptomatic phase and a significant drop upon disease progression. Treatment of severely immunodeficient HIV-infected individuals with the HIV protease inhibitor indinavir can reduce plasma HIV RNA levels and result in significant increases in circulating IL-16, RANTES, MIP-1 alpha, and MIP-1 beta levels, which can then act to inhibit further HIV replication. Because viral suppression does not depend solely on IL-16 or CC chemokines, the combined therapeutic potential of IL-16 and CC-chemokines may be more effective in suppressing HIV-1. In contrast, IL-16 antagonists may be useful in disease states where the proinflammatory functions of IL-16 are detrimental to the host, such as asthma, rheumatoid arthritis, systemic lupus erythematosus, colitis, atopic dermatitis, and multiple sclerosis. In a mouse model of allergic asthma, anti-IL-16 antibodies significantly inhibited development of airway hyper-responsiveness.
Figure 1. Nuclear-magnetic-resonance spectroscopic recreation of recombinant interleukin 16 (IL-16). This figure is a ribbon representation of residues 28–118 of rIL-16. The N and C termini of IL-16 are parallel structures that lie outside the core PDZ domain. The C-terminal 16 amino acids, centered around Arg116, are required for bioactivity and also define the epitope for neutralizing IL-16 antibodies. The core structure of IL-16 is a PDZ domain characterized by a GLGF motif, identified in pink, which is distinct from the bioactive site of IL-16.
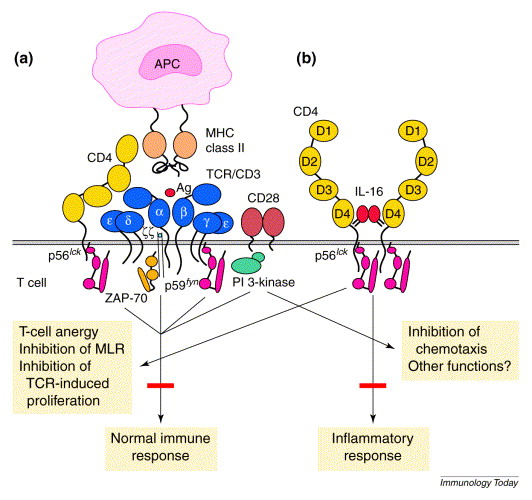
Figure 2. Hypothetical role of interleukin 16 (IL-16) and CD4 in switching from immune to inflammatory functions. (a) The role of CD4 in antigenic activation. During normal immune responses, CD4 associates directly with major histocompatibility complex (MHC) class II molecules via D1, with T-cell receptors (TCRs) via D2–D3 and with ZAP-70 via a p56lck interaction. This involvement results in enhanced TCR signaling and cellular activation. (b) The hypothetical role of CD4 after ligation with IL-16. CD4 is isolated from the TCR complex, oligomerizes and transduces TCR-independent signaling. IL-16–CD4-induced signaling results in a proinflammatory condition characterized by cell recruitment and cell-cycle progression, along with T-cell anergy to TCR stimulation. Abbreviations: APC, antigen-presenting cell; PI 3-kinase, phosphoinositide 3-kinase.
The discovery was made by two people:
http://www.bumc.bu.edu/pulmonary/people/faculty/williamcruikshank/
http://www.bumc.bu.edu/pulmonary/people/faculty/davidcenter/
Current directions of our research:
1. Role of IL-16 in Cutaneous T cell Lymphoma: is it a marker of the disease or a factor of pathogenesis?
2. IL-16 knockout mice – do they reflect a human autoimmune lymphoprolipherative syndrome?
3. IL-16 transgenic mice – a novel asthma model…
4. Early asthma development: nerve growth factors, wheezing and IL-16 – how are they connected?
5. Cocroach allergen as a trigger of asthma in early childhood: the epithelial cells differentiation, IL-16 and Notch signaling… (in vivo and in vitro models).
If you are interested, please do not hesitate to contact us!