Finished Projects
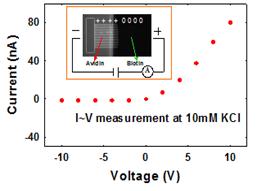
Finished Project I: Nanofluidic Diode
Ion and molecule transport control plays an important role for developing integrated micro-nanofluidic systems for biomedical applications. Here we demonstrated a nanofluidic diode that only allows ion flow in one direction. This nanofluidic diode was successfully realized by patterning positive charged protein (avidin) onto a biotin (electrically neutral) coated nanochannel using a diffusion-limited patterning technique. Under a forward bias, both cations and anions are accumulated in the center of the channel, resulting in significantly enhanced ion transport. While under a reverse bias, cations and anions were driven away from the center, forming a depletion regime and inhibiting ion movement. Our experiments suggest that nanofluidic diodes or other devices based on similar principles could find applications in control of pH and ionic concentrations and separation processes.
Ref:
1. R. Karnik, C. Duan, K. Castelino and A. Majumdar, “Rectification of Ionic Transport in a Nanofluidic Diode”, Nano Letters, vol. 7, pp. 547-551 (2007).
2. R. Karnik, K. Castelino, C. Duan and A. Majumdar, “Diffusion-Limited Patterning of Molecules in Nanofluidic Channels”, Nano Letters, vol. 6, pp. 1735-1740 (2006).
Schematic Diagram of a Nanofluidic Diode Diode I-V Characteristics

Finished Project II: Label-free Enzyme Sensor
We studied label-free electrical detection of enzymatic reactions, in particular trypsin proteolysis, using 1-D confined nanofluidic channels. When trypsin cleaves Poly-L-Lysine (PLL) coated on the surface of silica nanochannels, the resulting change of surface charge density can be detected by monitoring the ionic conductance along the nanochannels. Since the enzyme is not consumed during the reaction, detection of such surface enzymatic reactions is much faster than the detection of diffusion-limited surface binding reactions. We studied the sensitivity of this nanofluidic enzyme sensor and used it to explore enzyme kinetics in confined space. It was observed that the trypsin activity increases in such nanochannel devices due to geometrical confinement. The sensitivity of trypsin proteolysis also suggests that it may be possible to study enzyme activity with single-molecule resolution. Optimization of this nanochannel sensor could lead to a quick-response, highly-sensitive and label-free enzyme assay. Top
Ref: C. Duan#, MA. Alibakhshi, DK. Kim, CM. Brown, CS. Craik, A. Majumdar#, “Label-Free Electrical Detection of Enzymatic Reactions in Nanochannels”, ACS nano, vol. 10 (8), pp 7476-7484, (2016).
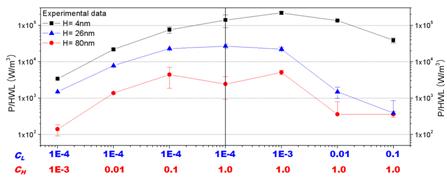
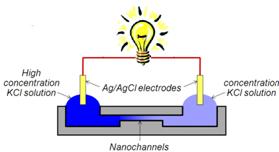
Finished Project III: Power Generation from Salt Gradient in Ion selective Nanochannels
When nanochannels were brought into contact with aqueous solution, the surface of nanochannels acquires charges from ionization, ion adsorption, and ion dissolution. These surface charges draw counter-ions toward the surface and repel co-ions away. Therefore, when an electrolyte concentration gradient is applied to nanochannels, counter-ions are transported through nanochannels much more easily than co-ions, which results in a net charge migration of ions. Gibbs free energy of mixing, which forces ion diffusion, thus can be converted into electrical energy by using ion-selective nanochannels. We experimentally investigated the power generation from 1-D silica nanochannels which were placed between two potassium chloride solutions with various combinations of concentrations. It is observed that the power generation per unit channel volume increases when the concentration gradient increases, while it decreases as channel height decreases. The highest power density measured was 221 kW/m3 and the best efficiency obtained was 30.7%. Our results confirm that inorganic nanochannels are suitable for such Gibbs- energy-to-electrical-energy conversion. A thin film membrane with imbedded thin nanochannel array could be a good candidate for large scale applications.
Ref: D. Kim*, C. Duan*, Y. F. Chen, and A. Majumdar, “Power Generation from Concentration Gradient by Reverse Electrodialysis in Ion-Selective Nanochannels”, Microfluidics and Nanofluidics, vol. 9, pp. 1215-1224, (2010).
Maximum Power Output
Schematic Diagram of Power Generation
Finished Project IV: Ion Transport in 1-D confined 2-nm Nanochannels
Transmembrane proteins often contain nanoscale channels through which ions and molecules can pass either passively (by diffusion) or actively (by means of forced transport). These proteins play important roles in selective mass transport and electrical signaling in many biological processes. Fluidic nanochannels that are 1–2 nm in diameter act as functional mimics of protein channels, and have been used to explore the transport of ions and molecules in confined liquids. Here we report ion transport in 2-nm-deep nanochannels fabricated by standard semiconductor manufacturing processes. Ion transport in these nanochannels is dominated by surface charge until the ion concentration exceeds 100 mM. At low concentrations, proton mobility increases by a factor of four over the bulk value, possibly due to overlapping of the hydrogen-bonding network of the two hydration layers adjacent to the hydrophilic surfaces. The mobility of K+/Na+ ions also increases as the bulk concentration decreases, although the reasons for this are not completely understood. These enhancements of ion transport in 2-nm nanochannels, in terms of both ion concentration and ion mobility, may find wide-ranging applications in single molecular detection, fuel cells and batteries.
Ref: C. Duan and A. Majumdar, “Anomalous Ion Transport in 2-nm Hydrophilic Nanochannels”, Nature Nanotechnology, vol. 5, pp. 848-852, (2010).
Finished Project V: Evaporation Induced Cavitation in Nanochannels
Cavitation, known as the formation of vapor bubbles when liquids are under tension, is of great interest both in condensed matter science as well as in diverse applications such as botany, hydraulic engineering and medicine. Although widely studied in bulk and microscale-confined liquids, cavitation in the nanoscale is generally believed to be energetically unfavorable and has never been experimentally demonstrated. Here we investigated evaporation-induced cavitation in water-filled hydrophilic nanochannels under enormous negative pressures up to -7 MPa. As opposed to receding menisci observed in microchannel evaporation, the menisci in nanochannels are pinned at the entrance while vapor bubbles form and expand inside. Evaporation is found to be governed by advective liquid transport, which leads to an evaporation rate that is an order of magnitude higher than that governed by Fickian vapor diffusion in macro and microscale evaporation. The vapor bubbles also exhibit unusual motion as well as translational stability and symmetry, which occur due to a balance between two competing mass fluxes driven by thermocapillarity and evaporation. Our studies expand our understanding of cavitation and provide new insights for phase-change phenomena at the nanoscale. Top
Ref: C. Duan, R. Karnik, M. Lu and A. Majumdar, “Evaporation Induced Cavitation in Nanofluidic Channels”, PNAS, 2012 .
Finished Project VI: Ion Transport in Graphene Nanofluidic Channels
Carbon nanofluidic structures made of carbon nanotubes or graphene/graphene oxide have shown great promise in energy and environment applications due to the newly discovered fast and selective mass transport. However, they have yet to be utilized in nanofluidic devices for lab-on-a-chip applications because of great challenges in their fabrication and integration. Herein we report the fabrication of two-dimensional planar graphene nanochannel devices and the study of ion transport inside a graphene nanochannel array. A MEMS fabrication process that includes controlled nanochannel etching, graphene wet transfer, and vacuum anodic bonding is developed to fabricate graphene nanochannels where graphene conformally coats the channel surfaces. We observe higher ionic conductance inside the graphene nanochannels compared with silica nanochannels with the same geometries at low electrolyte concentrations (10−6 M–10−2 M). Enhanced electroosmotic flow due to the boundary slip at graphene surfaces is attributed to the measured higher conductance in the graphene nanochannels. Our results also suggest that the surface charge on the graphene surface, originating from the dissociation of oxygen-containing functional groups, is crucial to the enhanced electroosmotic flow inside the nanochannels.
Ref: Q. Xie, F. Xin, HG. Park, C. Duan#, “Ion transport in graphene nanofluidic channels”, Nanoscale, vol. 8, pp 19527-19535, (2016).
Finished Project VII: Water Drying in Nanochannels
Liquid drying in nanoporous media is a key process in food, textile, oil and energy industries, but the corresponding kinetics remains poorly understood due to the structural complexity of nanoporous media. Here, we directly observe the drying process and study drying kinetics in single two-dimensional (2-D) nanochannels with height ranging from 29 to 122 nm. Two different drying behaviors are discovered in such nanoconfinements: continuous meniscus receding and discontinuous meniscus receding due to liquid bridge formation ahead of the meniscus, albeit similar drying rates. The geometry dependence of the measured drying rates is studied at different humidities and compared with a theoretical model considering liquid corner flow, liquid thin film flow, and vapor diffusion as contributors to the overall drying rates. Individual contributions from vapor and liquid transport inside the nanochannels to the drying kinetics are decoupled, and the water vapor diffusivity is successfully extracted. Our results show that both corner flow and vapor diffusion play important roles on water drying in nanochannels without sharp corners. Our findings further indicate that water vapor diffusion in nanoscale confinements can still be described by the classic Knudsen diffusion theory. These results provide new insights of liquid drying in nanoporous media and have implication in optimizing drying processes in industrial applications.
Ref: Q. Xie, S. Xiao, C. Duan#, “Geometric Dependent Drying in Dead-End Nanochannels.” Langmuir, 33 (34), pp 8395–8403, (2017).
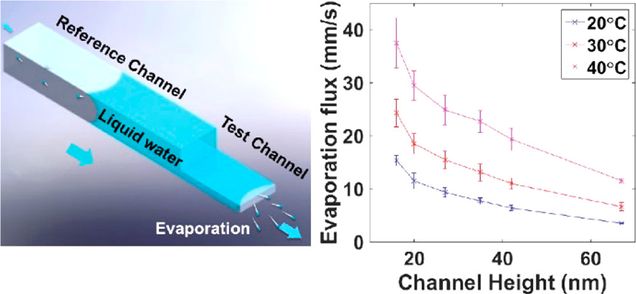
Finished Project VIII: Water Evaporation in hybrid-Nanochannels
Capillary evaporation in nanoscale conduits is an efficient heat/mass transfer strategy that has been widely utilized by both nature and mankind. Despite its broad impact, the ultimate transport limits of capillary evaporation in nanoscale conduits, governed by the evaporation/condensation kinetics at the liquid–vapor interface, have remained poorly understood. Here we report experimental study of the kinetic limits of water capillary evaporation in two dimensional nanochannels using a novel hybrid channel design. Our results show that the kinetic-limited evaporation fluxes break down the limits predicated by the classical Hertz–Knudsen equation by an order of magnitude, reaching values up to 37.5 mm/s with corresponding heat fluxes up to 8500 W/cm2. The measured evaporation flux increases with decreasing channel height and relative humidity but decreases as the channel temperature decreases. Our findings have implications for further understanding evaporation at the nanoscale and developing capillary evaporation-based technologies for both energy- and bio-related applications.
Ref:
- Y. Li, M. A. Alibakhshi, Y. Zhao, and C. Duan#, “Exploring Ultimate Water Capillary Evaporation in Nanoscale Conduits”, Nano Lett., 17 (8), pp 4813–4819, (2017).
- M. A. Alibakhshi, Q. Xie, Y. Li, and C. Duan#, “Accurate Measurement of Liquid Transport through Nanoscale Conduits”, Scientific Reports, 6, (2016).
Finished Project IX: Single Bubble Dynamics on Superheated Superhydrophobic Surfaces
This work explores single bubble dynamics on superheated superhydrophobic surfaces and the corresponding heat transfer mechanism of water pool boiling. The superhydrophobic surfaces are prepared by coating a thin layer of polytetrafluoroethylene on top of silicon substrates with electroless etched silicon nanowires. It is observed that a vapor film covers the whole superhydrophobic surface when it is immersed in water. Once the surface is superheated, single bubble forms and departs from the vapor film. We find that the bubble growth rate shows little dependence on surface superheat and applied heat flux at large superheats due to the presence of the vapor layer, which seriously limits the heat transfer from the superheated superhydrophobic surface. The bubble departure diameter is mainly determined by the static force equilibrium, but local disturbance and surface superheat also play important roles. There is a plateau of bubble departure frequency at large superheats, which is a result of the constant departure velocity limit. Accompanying the continuous bubble formation and departure, a smooth boiling curve is observed. The corresponding heat transfer coefficient (HTC) first increases and then slowly decreases as the superheat increases, showing a peak at a superheat of ∼50 K. Such HTC change is explained by the heat flux partitioning model, suggesting latent heat transport due to water vaporization and bulk liquid water convection after bubble departure contribute together to the heat removal on superhydrophobic surfaces. Insights gained from pool boiling on superhydrophobic surfaces might provide design guidelines for new surfaces excellent in heat removal or hydrodynamic drag reduction.
Ref:
- Y. Li, K. Zhang, MC. Lu, C. Duan#, “Single bubble dynamics on superheated superhydrophobic surfaces”, International Journal of Heat and Mass Transfer, vol. 99, pp 521-531, (2016).
- Y. Li, C. Duan#, ”Bubble-Regulated Silicon Nanowire Synthesis on Micro-Structured Surfaces by Metal-Assisted Chemical Etching”, Langmuir, vol. 31 , pp. 12291-12299, (2015).