Scientist Profile – Professor John White
Combining cutting-edge techniques to study how brain cells function together
John White, Ph.D. – Chair and Professor of Biomedical Engineering
The Neuronal Dynamics Lab, led by Professor John White (Center for Systems Neuroscience, Neurophotonics Center), uses optical imaging, computational, and electrophysiological approaches to understand what factors drive neuronal activity and synchronization within the brain.
Professor White—who will soon be concluding his 10-year tenure as Chair of Biomedical Engineering at Boston University (BU)—talked about two of his projects which explore stimulation strategies for the modulation of memories. He then discussed his path to becoming a neuroscientist as well as his new hobby mixing craft cocktails.
How would you describe your research and the goals of your lab?
Our goals are to bring novel engineering approaches into the study of the brain to understand neural function, including the way the brain computes, represents, stores, and retrieves information. We are also interested in the way that system breaks or malfunctions, and in what we might be able to do one day to create useful clinical approaches to repair broken brains.
Can you offer some examples of the tools your lab uses for this research?
One approach we use is optogenetics, which is a technique that allows us to control the activity of neurons with light by genetically modifying those neurons to express proteins that are sensitive to specific frequencies of light. We have also developed computer systems that allow us to perform various tasks based on real-time interaction, like delivering precisely timed stimuli to the brain. You can almost think of this technique as virtual reality for neurophysiology experiments. For instance, in some of our experiments, we immerse a real neuron in an artificial neural network that we designed, and by observing the neuron interact with that artificial network, we can begin to understand the principles by which neural networks self-organize.
Can you describe in more detail one of the studies from your lab?
I can describe one study we published that used the optogenetic approach I just mentioned. One of the PhD students in my lab at the time, Bahar Rahsepar (who has since graduated), got interested in the work of our BU colleague Steve Ramirez who, in the brain of a mouse, is able to tag cells which activate during a period of learning, then optogenetically stimulate those cells to drive behavior consistent with the recall of an associated memory. Bahar was also familiar with the work of our BU colleague Mike Hasselmo, who developed a theoretical computational model of memory. In this model, every 60 milliseconds or so, the hippocampus switches between encoding new memories and recalling old memories.
Our hypothesis was that we could use real-time computing to optogenetically stimulate tagged memory cells in the brain of a mouse, with precision timing, during the recall phase and then study the impact of that stimulus. We found that by stimulating cells during the recall phase in this manner, we could improve the recall ability. These findings, which we published about two years ago, have exciting implications. For instance, imagine a related type of therapy for patients with memory deficits, in which their brain is stimulated in this kind of precisely timed manner to improve their recall ability.
Have you conducted other experiments since then that use optogenetic stimulation?
Let me first just mention one experiment on our to-do list, which is to investigate the inverse of the study I just described. That is, are we able to stimulate tagged cells during the memory encoding phase—as opposed to the recall phase—and if so, how would that improve or otherwise affect the formation of a memory?
As far as more recent experiments involving optogenetic stimulation, we just published a study, this time led by PhD candidate Jacob Norman, in which we compared the activity of tagged cells involved in a particular memory to the activity of the surrounding memory cells. As in many of the optogenetic stimulation experiments we do, we first conditioned the mice with a brief static electrical shock, which produced a fear memory. Then, if we optogenetically stimulated the memory encoding cells associated with that memory, it would sometimes produce memory recall that we could observe behaviorally, because the mice would freeze during the time of stimulation. Other times, the stimulation would not produce a recall of the memory, in which case the mouse would not freeze.
Our goal was to observe activity in the recall cells when we stimulated the associated memory encoding cells, then compare that activity when stimulation produced successful recall of a memory versus when it did not (freezing vs not freezing). What we found, to our surprise, was that the activity level in those recall cells stayed the same whether stimulation produced a recall of the memory or not.
We then turned our attention to the surrounding “non-tagged” memory cells—the nearby cells not involved in the particular memory we were stimulating—and compared their level of activity when we stimulated the memory to their activity at baseline (that is, when we were not stimulating the memory). What we found was that, while there was a certain amount of activity in those surrounding cells at baseline, that activity level would drop when we stimulated the memory—the surrounding cells basically went quiet. In other words, when a memory is stimulated, it is almost as if the circuitry of the brain declares, “You surrounding memory cells need to shut up, because we need to hear what these particular cells are saying.”
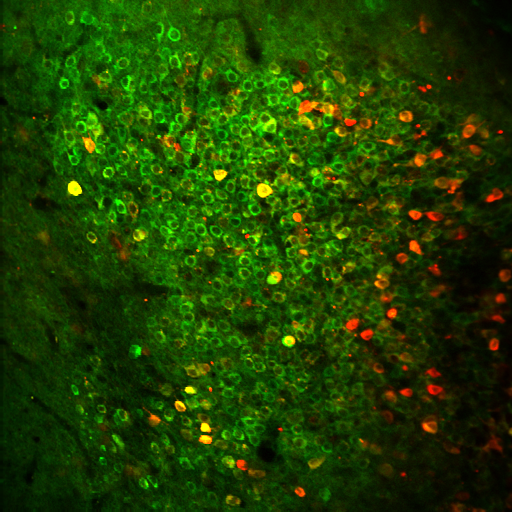
Did this recent study also involve collaboration with other BU faculty and labs?
Yes. In addition to working again with Steve Ramirez, we also collaborated with Anna Devor, who helped us set up the technology for simultaneous optical stimulation and recording, which was a tricky business.
I think both studies I’ve described are good examples of something BU does better than other universities, in that it attracts and hires scientists inclined to engage in highly collaborative research, and then has them work in these very interactive spaces—like here at the Rajen Kilachand Center for Integrated Life Sciences & Engineering—where people from different labs and centers are sharing resources, co-mentoring students, and just running into each other in the hallway and chatting about work. The studies I described would never have happened if Steve, Anna, Mike and I along with our lab teams were not all working and interacting in the same space.
Did you always know that you wanted to be a neuroscientist?
As a young child I was always fiddling with electronics, building radios and things like that, and so I figured electrical engineering would make a good career choice (I was a very practical kid). It was in college that I figured out I wanted to be a neuroscientist. I grew up in Louisiana and attended Louisiana Tech, which was a school that had mainly produced petroleum and chemical engineers for the oil industry, so I started off studying chemical engineering. Then I learned the school had this small biomedical engineering program, and so I switched to that.
Around the same time, I was also developing a strong interest in neuroscience, mainly through my own independent study. I never took a neuroscience class as an undergraduate; I just read on my own and took a couple of biology classes that included neuroscience segments. For graduate school, I attended Johns Hopkins because I wanted a strong engineering environment where they thought seriously about how to use instrumentation to study brain function and brain repair.
What are your hobbies outside of the lab?
My wife Sonia and I have a little cabin in Vermont on Lake Bomoseen, where we do a lot of kayaking and canoeing. A hobby I developed more recently is bartending. Scientists make good bartenders, it turns out, and I really honed my skills during the pandemic. If you have a good palette and can measure things precisely, you make a drink that tastes delicious. One source of inspiration for me is a documentary, called Schumann’s Bar Talks, that follows a famous craft bartender from Munich as he travels the world, sampling cocktails and discussing the diverse cultures of bartending.
Interview conducted and edited by Jim Cooney.