Scientist Profile – Professor John Ngo
Developing tools to study and reprogram cells at the molecular level
John Ngo, Ph.D. – Associate Professor of Biomedical Engineering
The Ngo Lab, led by Professor John Ngo (Biological Design Center) aims to achieve a better, multi-scale understanding of the molecular organization of life—and to help other scientists do the same—by developing tools to measure, probe, and even re-program how cells communicate with one another.
Professor Ngo shared a few examples of tools his lab has developed, including a sensitive new way for tracking and recording mRNA-based genetic messages one molecule at a time. He also explained how a childhood fascination with seashells helped put him on his path toward becoming a biologist, bioengineer, and gardener.
How would you describe your research and the goals of your lab?
I’ll start by saying that our approach to biology and bioengineering challenges us to make new tools, and lately we have been focusing on making two types of tools in particular. One class of tools helps us to visualize, track, and measure what molecules like proteins and mRNAs are doing inside cells, while the other class enables us to program cellular activities, such as how and when cells communicate with their neighbors. For instance, in studies we’ve published recently, we developed new ways to “turn on” gene expression within in cells, in response to chemical signals (such as drug molecules) or in response to physical stimuli (in the form of microscopic mechanical “tugs”). What makes these tools useful is that we’ve designed them in ways where they can be used to turn on any gene that we want in response to our chemical and physical cues. Tools like these could have important research applications, such as giving scientists new ways to study how cells work in tissues, as well as therapeutic applications, where they could be utilized to fine-tune the activity of cell-based therapies.
We try to pinpoint areas in the scientific landscape where the limitations of an existing tool set represent a research barrier or impose a bottleneck that hinders how quickly we can get to the next, deeper level of understanding in a given system. It’s at these junctions where introducing the right new tool could open things up and allow scientists to begin exploring new processes and asking new questions.
Can describe one or two examples of a tool your lab has developed?
I mentioned that one tool we developed allows a cell to turn on a particular gene when it senses a force. Some proteins in cells are force-sensitive, such that physically pulling on the protein can cause it to change its three-dimensional shape, transitioning it from an “off” state to an “on” state configuration in a mechanically-dependent way. Using this principle, we engineered force-sensitive proteins that, when pulled upon, can lead to the “turning on” of a specific gene. A neat part about this project is that we could also make predictions about how to adjust the proteins’ sensitivity, introducing mutations to fine-tune the force levels required to activate each of our various proteins. As I mentioned, we envision a range of applications for this tool. For instance, you could theoretically engineer a therapeutic cell that only delivers the desired therapeutic output when it encounters a specific physical signal—turning on when exposed to ultrasound, for example, or when the engineered cell encounters the unique physical environment of a solid tumor.
Another tool we developed recently involves visualizing individual copies of mRNA molecules, one at a time, in live cells. There are various ways to detect and measure mRNA activity, many of which involve crosslinking the cell’s interior and breaking up its membrane to label mRNAs inside. There are also methods for tracking the mRNA activity in living cells, allowing scientists to observe the molecules as they travel to different places inside the cell and perform their various functions. One prominent method for imaging this level of activity involves binding fluorescent proteins to the mRNA molecules to make them visible under a microscope. The limitation of this method, which our lab aimed to overcome, was that those fluorescent proteins were always fluorescent, regardless of whether they were bound to an mRNA molecule, making it difficult to distinguish between the mRNA molecules we wanted to see and free-floating protein copies in the surrounding area.
In a study that is set to publish in Nature Methods later this summer, we describe new tools to overcome the limitations of the existing toolset. Specifically, we designed a new protein that is fluorescent only when it is bound to an mRNA molecule. This results in a significant contrast between the tagged, illuminated mRNA molecules and the surrounding areas, making the tagged molecules much easier to see under a microscope, and allowing them to be tracked and enumerated in detail, in live cells, over time. We think these new tools will have many utilities, from investigating mRNA movements in neurons to understanding how altered gene expression leads to cancer.
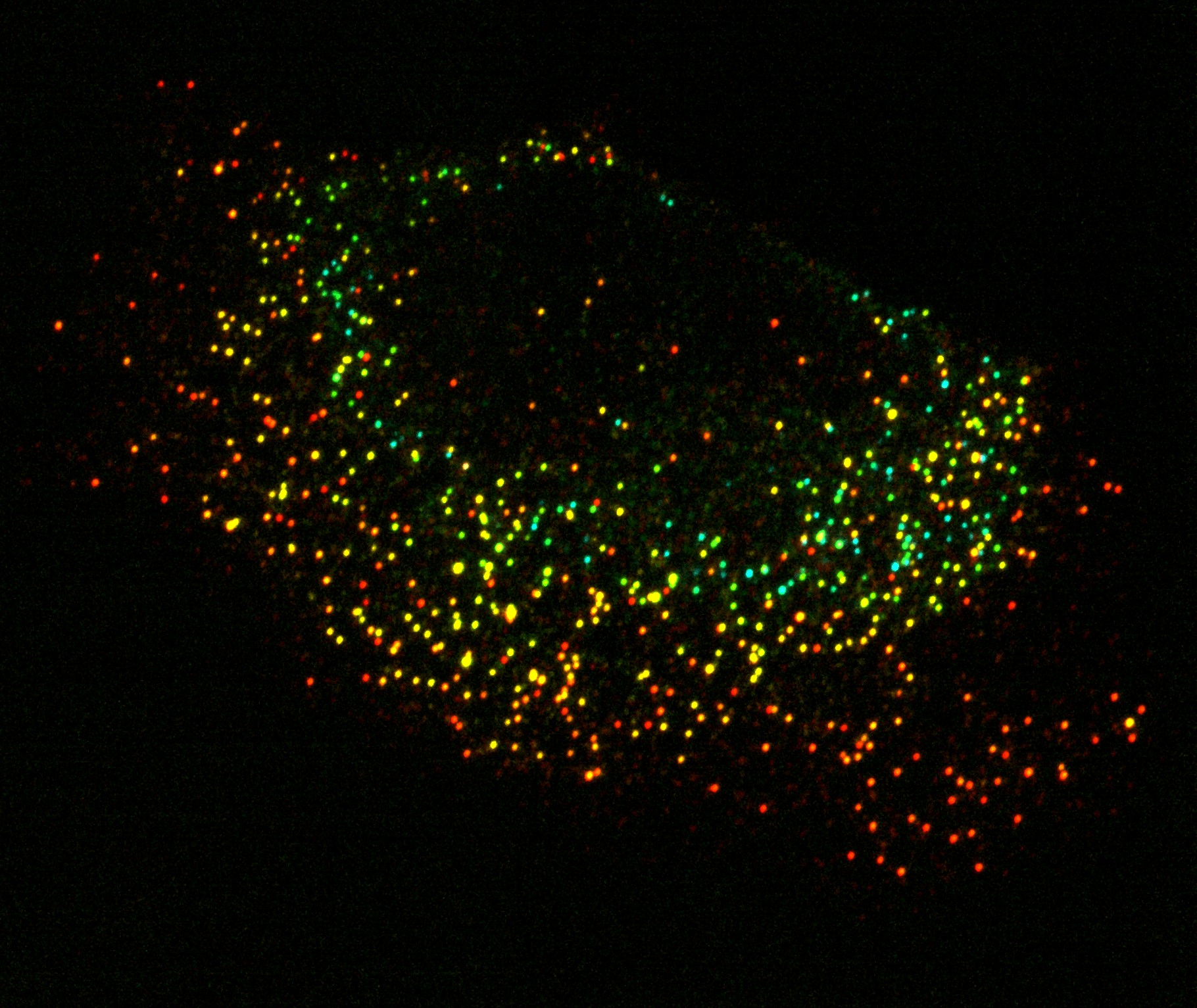
This confocal fluorescent micrograph shows the locations of tagged mRNAs as they exist within a cancer cell. In this image, each dot represents the location of exactly one copy of a given kind of mRNA. To give an impression of how the full set of these mRNAs are spatially distributed, the molecules are color-coded by depth, such that the RNA molecules furthest from lens show up as red spots whereas those closest to the lens appear as blue spots. The clarity of this image is made possible by a fluorescent protein, engineered in the lab of Prof. John Ngo, that only illuminates when it is bound to a target RNA molecule, thus rendering a strong visual contrast between the tagged RNA molecules and the area surrounding them. Image produced by Chris KuffnerDid you always know that you wanted to be a biologist?
One story I like to tell is that, when I was growing up in California, I loved seashells, and I recall one formative moment when I encountered an interesting looking tool that turned out to be a caliper for measuring the size of an abalone, which is a type of mollusk. At the time, I had no idea how a squishy creature like an abalone could grow something hard like a shell over the course of its life. As an elementary school kid, the mystery of it blew my mind!
It wasn’t until I was in college, many years later, that I learned that abalone possess genes encoding proteins that can bind dissolved calcium carbonate from oceanwater, allowing them to continually add new layers of mineral to their existing shells which, as I mentioned, grow externally alongside these creatures over their lifetime. How I came to learn that, it turns out, is that I began working for the researcher responsible for figuring out the molecular requirements for farming abalone—before his research, the only way you could use abalone as a source of food was to catch them in the wild. By the time I started working in this professor’s lab, he’d transitioned from studying abalone spawning to investigating the biochemical mechanisms of the organism’s ability to grow its shell.
In college I’d started as a political science major, but I quickly realized it wasn’t for me and so I switched to biochemistry, yet it wasn’t really until I started working in a lab that everything clicked. From that point onward, I viewed every class I took as way to become a better scientist and researcher. For me, getting to do research was really transformational—and I’ve been hooked ever since!
What are your hobbies outside of the lab?
I really enjoy gardening. I grow tomatoes in the summer, and I like experimenting with new methods, such as adjusting when I start planting, or how much exposure to sun they get, or when I water them. Also, like so many people in my neighborhood, I grow peonies. They’re exciting because they only bloom for about two weeks, so you’ll wait all year and then starting around late May you’ll get this brief burst of color. My partner and I will walk around the neighborhood when the peonies are in bloom, taking note of the many varieties that exist. I’m always intrigued when I encounter a new type of peony, especially those where you can just tell they are hybrids of two types we are familiar with and have seen before.
Interview conducted and edited by Jim Cooney.