Congratulations to NPC Professor Anna Devor and her collaborators Anton Arkhipov at the Allen Brain Institute and Li-Huei Tsai at MIT for receiving an NIH BRAIN Initiative award R01NS122742 to study cell-type-specific mechanisms of Patterned Sensory Stimulation (PSS)! PSS is a non-invasive technique for manipulating brain activity and states, employing, for example, periodic visual or auditory stimulation, which has been shown to cause widespread changes in properties of neurons, non-neuronal cell types, and vasculature. Through these changes, PSS can lead to improvements in cognitive function and activation of neuroprotective pathways in animal models of neurodegeneration. In this collaborative project, bio-realistic modeling and experiments will be used to investigate how cell types and circuit properties in the brain mediate the entrainment of neural activity and modifications of the states of different neuronal and non-neuronal cell types under PSS. Dr. Devor and her team at BU will focus on specific vasoactive neuronal cell types to enable modeling of vascular dynamics driven by neuronal circuit activity induced by PSS. These studies will provide the mechanistic understanding necessary for effective applications of PSS for future potential scientific and therapeutic purposes.
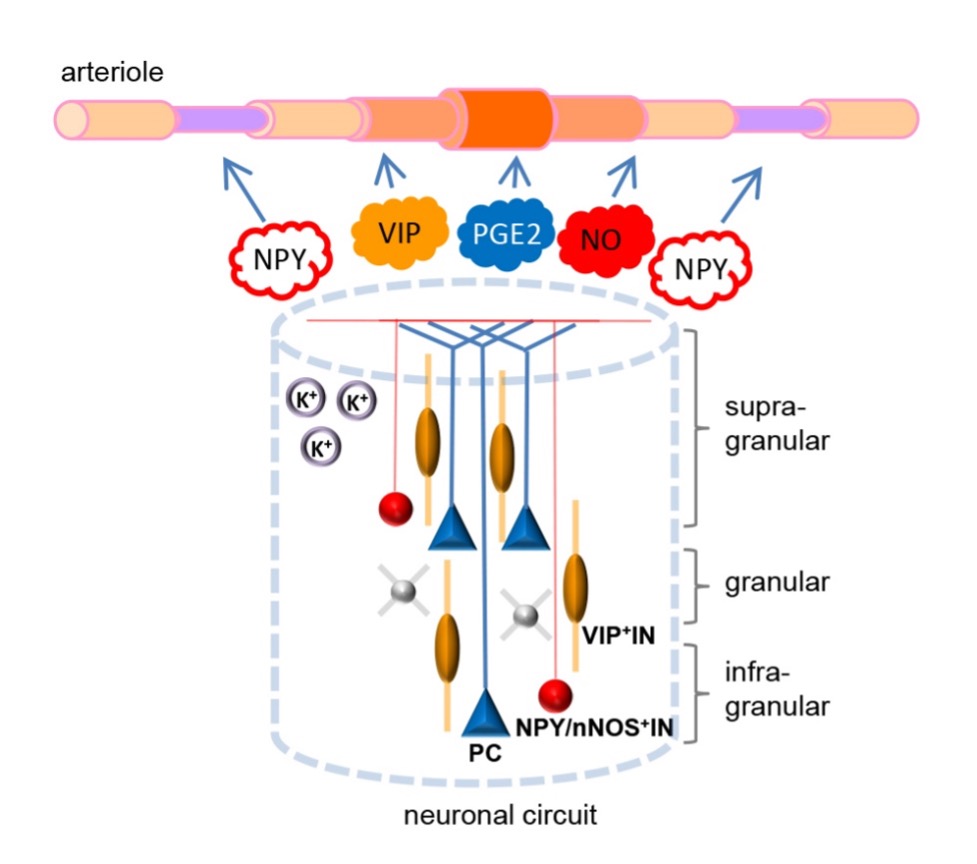
Cell Type and Circuit Mechanisms of Non-Invasive Brain Stimulation by Sensory Entrainment Patterned sensory stimulation (PSS) is a non-invasive technique for manipulating brain activity and states, typically employing periodic light flicker or auditory tones presented at regular intervals. We and others have recently shown that PSS at certain frequencies (centered at 40 Hz) causes widespread neural entrainment and state changes in non-neuronal cell populations (including, e.g., effects on the activity of microglia and on vasomotion), improvements in memory and cognitive function, and clearance of markers of neurodegeneration in animal models of brain disease. These observations suggest a strong potential of PSS for non-invasive brain stimulation applications in basic science and as a therapeutic tool. To enable such applications, however, it is important to know the mechanisms mediating the complex effects of PSS on neurons and non-neuronal cells. These mechanisms are poorly understood. In this project, we systematically investigate mechanisms of PSS by dissecting how cell types and circuit properties in the brain mediate the entrainment of neural activity and modifications of the states of neuronal and non-neuronal cell populations, with the focus on the mouse cortex as a model system. The central component of this project is a systematic modeling effort, relying on our recent progress in integrating diverse structural and functional data into highly detailed, bio-realistic models of the mouse cortical circuits. These models will be applied and refined to simulate the effects of PSS at the level of a single cortical area (primary visual cortex) and the whole mouse cortex. We will also develop models of coupling from the activity of different neuron types to non-neuronal cells, providing insights into the effects of neuronal entrainment to PSS on, e.g., microglia and vasculature. These modeling efforts will go hand-in-hand with electrophysiology recordings in awake mice, accompanied by chronic and acute perturbations (using chemogenetics and optogenetics). In multiple iterative stages, modeling predictions regarding the roles of excitatory and inhibitory (e.g., PV, SST, VIP) cell types in different cortical layers on the entrainment to PSS will be tested experimentally, and models will be refined to match data. The project will also characterize transcriptomic and epigenetic responses to PSS in different cell types, which will be correlated with circuit effects revealed by simulations and perturbative experiments in vivo. The results of these studies will provide a rich description of molecular, cell type, and circuit mechanisms mediating the PSS effects, which will be crucial for future rational development of applications of this brain stimulation technique. Besides the knowledge, this project will also provide highly biologically realistic, ready- to-use computational models applicable for studies of PSS and other phenomena, which we will freely share with the community.