 |
Lesson 3 Forces and Energy
Essential Concepts:
Matter can be charged positively and negatively
Atoms can be broken up into positively charged protons, neutral neutrons, and negative electrons
Like charged objects repel each other, unlike charged objects attract each other
Example: static cling – note this is not magnetic
There are four basic forces: nuclear (strong and weak), electromagnetic, and gravity
Types of energy include; heat, light, sound, electrical
Background:
Atoms
Š Smallest unit of matter; can’t be broken up using normal means (non-nuclear)
Š But the atom has components also: Proton, Neutron, Electron
Š Different elements have different numbers of Protons, Neutrons and Electrons—you can tell the name of the element by counting the protons and consulting the Periodic Table.
Š But what is the structure and what holds them together?
Atomic Structure
Š Protons and Neutrons-- 1000 times as massive (1000 times heavier) than electrons
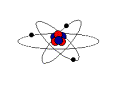
Š Classic Model: Planetary model – protons and neutrons are stuck together in the nucleus, and electrons orbit very quickly around the nucleus
Š Another analogy: Protons and Neutrons are stuck together in the nucleus – like a bunch of students playing cards, the electrons are like very active students running past the game, but they’re interested in the game, so they come back to see what is happening. They don’t stop—they just keep on running past and coming back. (This analogy is better because while the electrons are somewhat confined to specific regions around the atom, called “electron clouds”, these regions are not limited to the flat elliptical orbits of planets)
Š But what makes it all stick together? Why do the electrons need to stay around the nucleus? Why does the nucleus stay in one piece?
Four Forces
Š There are four fundamental forces: Strong, Weak, Electromagnetic and Gravity.
Atoms use the first three to stick together
Electromagnetic:: the force that deals with electrically charged objects
Š Objects with like charge repel each other (Electrostatic repulsion), objects with unlike charge attract each other (Electrostatic attraction)
Š Protons are positively charged, electrons are negatively charged (neutrons are neutral)
Š Therefore there is an attractive electrostatic force between electrons and protons: even though the fast moving positive electrons are whizzing all over the place, they stay relatively near the positive protons in the nucleus
The Strong and the Weak forces hold protons and neutrons together in the nucleus
Š Electrostatic repulsion between positively charged protons should cause the nucleus to fly apart—Remember, if you put a plus and a plus close together then they should fly apart!!
Š But at the very small size scale of the nucleus, the strong and weak forces are much stronger than the electromagnetic force, and hold the positively charged protons and the neutral neutrons together
Gravitational force is the very weak attractive force between all matter
Š All objects (you, me, atoms, chairs, neutrons, Sun, Earth, Moon, marshmallow, everything) attract each other Gravitationally
Š But this force is very weak compared to the other forces. It takes a HUGE mass, like the size of a planet, for it to be felt, whereas you can feel the force of charged objects the size of your finger
Types of Energy:
Š Energy is the ability to cause motion or create change.
Š Energy is everywhere you look: Types of energy include; heat, light, sound, electrical
Goal 1: Teachers will continue to think about the atom
Goal 2: Students will be introduced to the idea that there are ingredients that make up each atom: Protons, Neutrons and Electrons and that Protons have a plus charge and Electrons have a negative charge
Objectives:
Students will design and create a model that illustrates the atom
Materials:
Yarn
Blue “+” sheets and Red “-“ sheets and yellow blank sheets
Lesson 3 Forces on board sheet
Procedure:
1. Begin class by showing the students a large lego block. Ask them what it is. The correct answer is: a lego block. What did we use it for in class the other day? (to represent an atom or element) What are molecules? (groups of atoms joined together)
2. Let’s think about atoms. Scientists actually have figured out what atoms are made of and are working to better understand a very interesting puzzle. We won’t solve this puzzle this year—but would you like to know about it?
3. The puzzle is…what holds the atom together. So let’s think about things that are stuck together or held together. Can we think of some examples? (Glue…complicated has to do with molecules mixing together; magnets…what do we know about them…positive, negative, opposites attract; earth and moon…gravity) Try to get to magnetism and gravity.
4. Write on board: Atoms Electromagnetism Gravity
Under Electromagnetism write: “+” and “–“ Discuss opposites attract, same sides repel (Don’t worry-we’ll get to the electricity part soon—and we’ll find that electricity and magnetism are connected!!)
And under Gravity write: “Big” and “small” Discuss how all matter attracts all matter, although this force is so weak that it requires BIG objects for it to be noticed. So, the small object is also attracting the big one. Ex.:Sun and planets
Consult “Lesson 3 forces on board” sheet to see an example
- That leaves us with atoms. Here’s what scientists know about the atom: inside the atom are 3 kinds of stuff: Protons, Neutrons and Electrons. In the middle are the positive Protons and they like to stick to each other and the Neutrons. Draw a diagram of a bunch of circles with “+” inside stuck to a bunch of empty circles. (Remember: Protons are Positive, Neutrons are neutral )
Orbiting around these are the Electrons—draw several circles with “-“.in them on an orbital path around the protons and neutrons. Draw the orbital path so that it goes under the Electromagnetic space on the board. Do not label them other then with positive or negative symbols so that you will be able to assess the students later.
- Can anyone spot the puzzle? Look at the diagram. Here’s a hint: remember that magnets like to stick together in what way? (positive to negative) How do they not like to stick together? (positive to positive or negative to negative) So what is weird about our diagram of the atom? Does it make sense that the electrons would like to stay around the protons? (yes) What about all of those “+” that are stuck together? That is the puzzle. Scientists call the forces that keep those guys together the strong and weak forces. Write “Strong” and “Weak” under Atoms on the board.
- Draw in the electrons so that they are under the Electromagnetic section of the board -- since they are attracted electromagnetically to the Protons and Neutrons. It is only the nucleus that is held together by strong and weak forces.
- During the next several weeks, we will be talking about what electrons do and so it would be helpful if everyone could remember the parts of the atom. Lets try to make some models of an atom. You will need to be able to identify the following (write on board):
Protons, Neutrons Electrons positive negative neutral
In small groups of 6, use these colored sheets and yarn to make “charge necklaces” and form yourselves into an atom. This will be a hydrogen atom (2 protons)
- When the students have finished, pick one student from each group to show where each of the terms are in their model. When all groups have been asked to do this, ask one group to stay up while others sit down. Explain that scientists used to draw atoms like this—kind of like a solar system. For example, let’s change the kind of atom we have. Bring up one Proton, Neutron and Electron from another group sitting down and add them to the group already up there so that there are 3 of each(this is Lithium—3 protons).
The Protons and Neutrons will hold hands with the other Protons and Neutrons, while the Electrons can become a new orbital around the center group. Stop them from moving and draw everyone’s attention to just one Electron. Scientists now like to show atoms in a new way. Each electron is actually flying around the center group—we kind of know where it will be, but it moves really fast. So, if we could have this student fly and we took away the floor and ceiling, we would have a better model. These electrons get to have all sorts of interesting activity. In the next few days and weeks, we will be following these electrons around!
Activities: discussion, Making Atom model
Forces:
Atoms Electromagnetism Gravity
![]()
![]()

![]()
![]()
![]()
![]()
![]()
![]() +
+
![]()
![]()
![]()
![]() + +
Big Small
+ +
Big Small
Strong &
Weak
![]()
![]()
+ (Positive) -- (Negative)
Protons
Neutrons
Electrons
Positive
Negative
Neutral
Lesson 3 forces on board sheet