Lesson 1 The Water Cycle/States of Matter
Essential Concepts:
Water cycles on Earth in different forms
States of matter of water
Comparison of properties of solids, liquids and gases
State transformations by adding and removing heat
Water expands with negative temperature change
Background:
Water Goes Around
· Water continuously cycles between parts of the earth
· Liquid water evaporates into water vapor due to the addition of heat
· Water vapor condenses back into a liquid when heat is taken away
· This liquid precipitation falls back to earth (due to the force of gravity) as rain, snow, hail, etc..
· Some water will “run off” earth features so as to reach a lower place
Molecules Move
· All atoms and molecules are constantly vibrating or moving
· This means that even solid objects have a small rate of vibration
· When molecules are heated, they move around more
· Some Molecules like to stick together—especially at low and room temperatures
· However—if you heat them enough, they will become so active that they will split apart
An example of this is water. At room temperature, water molecules are reasonably mobile, while still staying together. At cold temperatures, these molecules are not as active and assume a crystalline form. At high temperatures, these molecules split up and become parts of a gas.
Goal 1: Students will understand the terminology and movement of the water cycle
Goal 2: Students will begin to understand molecular movement and will recognize states of matter
Objectives:
Students will:
- Understand and memorize the terms: evaporation, condensation, and precipitation
- Act out the part of a water molecule at different temperatures.
- Recognize that ice crystals form in groups of six (like a snowflake)
- Count the number of “molecule” students that can fit in a specified space at different states of matter (this will return some day as “Density”) to understand why ice floats.
- Observe demonstrations of the water cycle and states of matter.
Materials
Water Cycle demo:
Hot plate, ring stand, pan, pie plate, ice, water
States of matter Demo:
Hot plate, pan, flask, balloon, water,
Outdoor roleplay of States of Matter:
2’x4’ board or piece of cloth
stick (to “change” temperature
2 concentric circles on blacktop(class should fit into smaller one)
Water Cycle Quiz
States of Matter Quiz
Procedure:
Water Cycle
1. Discussion: Review water cycle on white board. Suggest possible ways to remember the terms. For example: When you take a Can of soda out of the fridge, you will see “Candonsation” and Can, Condensation begin with the letter “C” which also starts the word, “Cloud.” Or take a “Sip” from some precipitation.
- Show Demo to make it rain in the classroom. (Heat water to create vapor that will come into contact with flat metal surface cooled by ice.) Be wary of too much ice tipping over your ringstand—use 2 stands with clamps to be extra sure.
- Discuss: What is the difference between a picture and a diagram?
- Have students diagram and label:
· the water cycle in nature (draw a mountain, pond, and cloud on the board as a model
· the demonstration at the front of the room
using the following labels (write on board):
Precipitation, Run-off, Evaporation, Condensation, hot, cold, water vapor(steam), ice, water
- Finish diagrams for homework.
States of Matter
- Set up demo so that some water trapped in a flask will evaporate quickly, causing a balloon that has been stretched over the opening to inflate. This will take several moments.
- Ask the students what will happen to the balloon and why.
- Review the terms used in the water cycle lesson. Review states of matter for water (solid-ice, liquid-water, gas-water vapor). How might we change the gas into a solid? Discuss.
- Discuss: small pieces of water are called molecules. Molecules are always moving—hard to imagine for solids, but gas and liquid seem reasonable.
- Remove flask from pan of boiling water after balloon is inflated—what will now happen to the balloon—why?
- When flask cools, bring it and the students outside and show them how condensed water will flow down the sides of the flask to join the larger portion of water. Why is this? (gravity, water likes to stick to water) (Optional—before going outside, have students push drops of water on wax paper close enough to one another so that they form single drops. This will reinforce the idea that water stick to water. You can reference this or also ask them to remember when the drops fell as precipitation in the water cycle demo.)
- Inform students that they will be water molecules in a glass flask and ask them to stand inside the inner circle. The temperature will be room temperature so they are a liquid. They will need to hold hands because water molecules “like” to stick together and they can’t leave the glass flask circle(just like the real water can’t get out of the flask). Illustrate this with the real flask and water. Notice that they are free to move around (vibrate) as individuals, even though they are connected in a chain.
- Put a wooden or cloth rectangle under the students and have them count how many “molecules” or persons can stand on it. (around 8)
- Now comes the real imagination part—you will use your magic temperature stick to raise the temperature. They will need to stop whatever they are doing as soon as you say,”stop” —(not “freeze”)—this is important for management reasons. As soon as the temperature is raised they will start to have more individual movement and will be able to gently separate from the other molecules. The students can move into the larger outer circle as they are now able to enter the balloon. Tell them to “stop” and put the cloth or board under a student. Ask the kids to count how many “molecules” can fit on the board. (1 because they are moving around so much that they bump each other away from their space)
- Now “lower” the temperature to room temperature and have them condense back into water drops and into a group of water in the small circle.
- Now “lower” the temperature to below freezing. Explain that water likes to form crystals with 6 molecules (someone might say “like snow” or prompt them). Have the six students straighten out their arms and hold them taut pointing into their own small circle. Spread them out so that the crystals are touching to form an ice block. Notice that some of them are now outside of the small circle(the flask). What does this mean for the glass flask? (it has broken) Put the board under several students and ask them to count the “molecules. (2)
- Now ask them to remember how many molecules fit on the board when they were water(8). Which has more molecules in this space—ice (2) or water(8)? Which floats above which in real life? What about gas(1)? Does this match their real-life experiences?
- Return to the classroom and select students to review how the molecules act during different states of matter. Review fact that all molecules move—even the solid ice molecules were shivering a little. (“I’m so slowed…”)
- Homework: review concepts (terms and diagrams) for quiz on following day.
Note on States of Matter Quiz:
Density is very hard for 5th graders to understand. The idea that there are different amounts of molecules in a certain amount of space is difficult—even when they are standing on the board. You may want to remove the third question from the quiz for this reason.
Optional Demo: Frozen iron sphere
Using liquid nitrogen and a demonstration iron sphere, show that ice is stronger than iron. Put water into sphere, screw cap on tightly!! Put into plastic container of liquid nitro and cover with a plastic trash barrel. Wait.
Optional Demo2: Moving Molecules
To show that warm molecules move faster than cool molecules, put 2 drops of food color into very hot water and time how long it takes for the color to be absorbed by the water. Next put an identical amount of food color into very cold water and time how long it takes for the drops to be absorbed. It should take less time for the warmer, faster-moving molecules to spread out into the water.
Activities: discussion, diagram drawing, acting as molecules
Name ___________________________________ Class ______ Date ____________
Science Quiz
1. Use the following words in a diagram of the water cycle:
Evaporation Condensation Precipitation Run-off
2. Use the following words and phrases in a paragraph or diagram that explains the experiment that is at the front of the room:
Evaporation Condensation Precipitation Heat Source
cools off heats up forms drops of water
forms water vapor (steam) Ice Water
Name ___________________________________ Class ______ Date ____________
Science Quiz
1. Connect the dots (or don’t) to show how water molecules are connected in each state of matter:
Solid (Ice) Liquid (Water) Gas (Water Vapor)
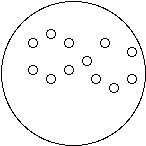
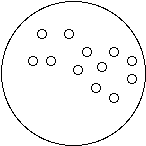
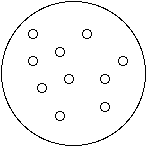
2. All molecules are always vibrating or moving.
Circle one: True False
Extra Credit:
3. Why does ice float on top of water?