Preventing an Antibiotic Apocalypse
The business model for drug innovation is broken — universities key to figuring out fixes, says health law prof
By: Sara Rimer
 Kevin Outterson is a leading scholar on the economic and legal global framework needed to combat resistance and keep antibiotics available for future generations. Photo by Jackie Ricciardi.
Kevin Outterson is a leading scholar on the economic and legal global framework needed to combat resistance and keep antibiotics available for future generations. Photo by Jackie Ricciardi.
When Kevin Outterson, a professor of health law at Boston University’s School of Law, began studying antibiotic resistance a decade ago, few people outside academia seemed interested. In the past year, however, President Obama and British Prime Minister David Cameron and other European leaders have joined global health officials, physicians, scientists, and scholars like Outterson in calling for urgent action to combat the rapid spread of antibiotic-resistant germs.
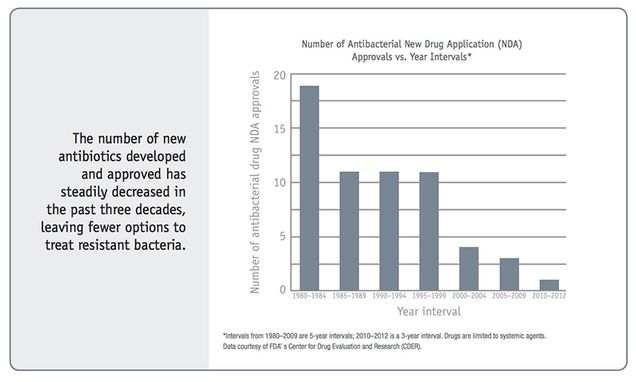
In its first such survey, published in 2014, the World Health Organization (WHO) warned of a looming post-antibiotic era, in which common infections and minor wounds that have been treatable for over half a century could once again prove fatal. In the US alone, more than 23,000 people die each year from antibiotic-resistant infections, according to the Centers for Disease Control and Prevention (CDC). A similar number die in Europe, and in southeast Asia one child dies every five minutes from such infections, according to health officials.
Outterson, who co-directs BU’s Health Law program, is a founding member of the CDC’s working group on antibiotic resistance and a leading scholar on the economic and legal global framework needed to combat resistance and keep antibiotics available for future generations.
He is the co-author of an article in the February Health Affairs, the top US health policy journal, contending that the business model for antibiotic innovation is broken: While a new pipeline for effective antibiotics is urgently needed, there is little financial incentive for companies to develop new antibiotics. His article proposes prizes and other government-funded financial incentives to encourage innovation, vaccines, and point-of-care diagnostics for infections. Outterson and his co-authors emphasize that resistance is a global ecological problem, requiring not just new drugs but also aggressive conservation to stop us from wasting these precious drugs. They write that overuse in agriculture is only one way that antibiotics pollute the environment and create evolutionary pressure for resistance.
Outterson, who is the N. Neal Pike Scholar of Health and Disability Law, spent a sabbatical at the Royal Institute of International Affairs at Chatham House in London, building relationships with European researchers. He has produced several major reports for the think tank, including one in 2014 and another published in February 2015. He has also joined a global research team known as DRIVE-AB (Driving Re-Investment in Antibiotics), funded by the European Union. In September 2014, the day after President Obama issued an executive order on addressing antibiotic resistance, Outterson testified before Congress as an expert on building better business models for antibiotics.
BU Research sat down with Outterson over lunch at the School of Law to discuss his ideas for antibiotic innovation; Obama’s FY2016 budget allocation of more than $1.2 billion to address antibiotic resistance; and why he thinks major research universities like BU are the key to preventing an antibiotic apocalypse.
BU Research: You and your co-authors write that the pharmaceutical industry’s traditional price-volume model—the need to sell drugs at high volume and/or high prices—doesn’t work for antibiotics, and many companies have abandoned the business. Why have they left?
Outterson: Most large drug companies have pared back their antibiotic research because they can make a lot more money selling other drugs. There are many generic antibiotics and they’re highly substitutable, especially when we lack good diagnostics to guide therapy. In contrast, the hepatitis C drug Solvadi is $1,000 a pill. A course of treatment costs $80,000, but it’s still a bargain. You’re curing hepatitis C and saving people’s lives. Solvadi was a breakthrough treatment and the reason they are able to charge such a high price is that there were no alternatives.
For antibiotics, they can save people’s lives, but there are so many alternatives and many of them are generic. When you go into a hospital, one of the major antibiotics they might give you is vancomycin. It was introduced in 1958 and it’s under $200 for a course of treatment in a US hospital. So it could save your life for $200. It’s the best bargain in the hospital.
If you brought a new antibiotic to market for use in hospitals, the way health care reimbursement works, you have to compete against vancomycin. How much can you charge for this new antibiotic? If the company charges $3,000, that is fifteen times the price of vancomycin. Many hospitals will choose the cheaper drug and only use the new one if vancomycin doesn’t work. For antibiotics, the market works almost too well: prices are kept low by cheap generic competition, which drives companies away.
You write that another reason the price-volume model doesn’t work for antibiotics is because important public health measures—like using the existing antibiotic if it’s effective, and not the new one—end up discouraging volume of sales and becoming another market disincentive.
If the older, cheaper drug is effective, why not use it? From the public heath standpoint, that’s the correct answer: Let’s use up these other drugs first, then when they’re worthless we’ll move on to the next new drug. For public health, good stewardship is the right answer. For the company, it means the new drug doesn’t sell for the first decade.
You have to find another way to reward the company based on the value of the drug, not the volume of sales. We’ve been working on this for more than two years at Chatham House.
Can you elaborate on the antibiotic innovation/iPhone metaphor you often use in talking about this? What do iPhones have to do with antibiotic development?
Imagine you are Apple and you are ready to sell the latest iPhone, but the experts insist that no one is permitted to buy the iPhone 6 until all of the existing phones don’t work anymore. No sales until the iPhone 3s, 4s and 5s and every Android phone are completely obsolete. If cell phone markets worked that way, Apple would stop selling them. You’d kill the innovation system. Would we ever have gotten the first iPhone if that were the system? No.
That is the situation faced by companies bringing new antibiotics to market, except that the experts are absolutely correct to insist that we conserve new antibiotics through careful stewardship. Resistance makes antibiotic markets behave in remarkably problematic ways.
You’ve also written about another problem—that we don’t have good diagnostics for bacterial infections and that we also need increased funding for point-of-care diagnostics.
In a perfect world, the doctor would know what infection we have and treat it with the best drug. With cancer, the treatments are increasingly targeted to the specific genetic profiles of the patient and the cancer. Solvadi is $1,000 per pill. They only give it to people clearly diagnosed with hepatitis C.
When it comes to bacterial diagnostics, we are still in the 19th century. Most patients are still treated ‘empirically,’ which is a code word for the best guess without really knowing. With lab-based diagnostics, the physician takes a sample of the infected material, puts it on a petri dish and lets it grow for 48 hours. Then a microbiologist looks at it. By the time the diagnosis comes back, the patient could be dead. So in the absence of effective diagnostics, physicians treat now and understand later. In these circumstances, they need a broad-spectrum antibiotic while waiting for the lab results to come back.
The gold standard should be a point-of-care diagnostic that would be both specific and sensitive and would tell the physician what they needed to know in 15 minutes. We do not have that test. Until we do, physicians have to shoot first and ask questions later.
Fortunately, the UK government has now sponsored a £10 million prize for exactly that diagnostic. I’m excited to serve on the Advisory Panel for this new Longitude Prize and we hope to see some BU teams enroll and give it a try.
Haven’t scientists been warning about the possible misuse of antibiotics for years?
Yes, Alexander Fleming in his 1945 Nobel Prize lecture said that if we overuse these drugs and prescribe them inappropriately, resistance will develop, destroying the drugs. You should read his Nobel speech. It’s remarkably prescient. If you expose these bacteria to doses of antibiotics that don’t kill them, you select for resistance. He understood evolutionary biology. In one sense, this is very discouraging: We have understood the problem from the very beginning, but haven’t taken effective action in the ensuing seven decades.
So people didn’t pay attention to what Alexander Fleming said?
There’s a book that Scott Podolsky at Harvard Medical School just wrote called The Antibiotic Era. It’s an excellent book. He describes how the US in the 1950s was just wasting antibiotics. Companies ran amazing campaigns to promote antibiotics. Even though they knew the common cold was viral and would not respond to antibiotics, companies sold combinations of drugs to clear your stuffy nose with antibiotics. It was a way to make money. Very short-term thinking.
Resistance is exactly the sort of problem that is too complicated, too interdisciplinary, to be solved by anyone other than academic researchers working together in universities. I think the very purpose of a modern research university is to tackle incredibly complex global problems like resistance.
You talk about how antibiotics are wonder drugs that have saved millions of lives and you were quoted in The New Yorker magazine last August saying that antibiotic resistance has the potential to make everything about the way we live different. Can you give us some examples?
If superbug bacteria become endemic in US hospitals, it will shake our health care system at its foundations. Why would you get a hip replacement, or a cardiac stent, or do anything else in a hospital that wasn’t a life-saving emergency, if you knew these infections could kill you?
We have MDR (multidrug-resistant) gonorrhea in the US and around the world. Only one drug remains against the nastiest strains. On the CDC’s threat assessment list in 2014, gonorrhea made the list of the three most dangerous resistant pathogens. We’re close to having no effective treatment for gonorrhea.
We’re almost back to the 1930s. The same is true with XDR (extensively drug-resistant) tuberculosis. Our best treatments for malaria are showing signs of resistance, too. For a number of serious hospital infections, we are down to the last-ditch treatment.
Right now, antibiotic resistance is a very slow moving train wreck. It might take another decade for this to become a true disaster, or it might be tomorrow. Boston has several underwater tunnels. If we stop investing in tunnel maintenance, one day the tunnels are going to fill with water—we know it will happen, but don’t know when.
You worked for two private law firms before you switched to academia. Who were your clients?
Some of my favorite clients were academic medical centers.
It must have been interesting.
When you practice law, you learn a lot from your clients, if you listen carefully.
What did you learn that you use in your work on antibiotic innovation and how to fix the broken model?
The first question anyone asks in US health care is “How will this be paid for?” The key is reimbursement. A lot of the work I did in private practice was to help clients design services in US hospitals that would be reimbursable under Medicare. For antibiotics, much of my work focuses on how health systems around the world reimburse for antibiotics, because payments and incentives drive behavior. For example, for many years Chinese hospitals were underfunded by the government, but they were allowed to keep any profit they made by selling antibiotics. For a number of years, a very significant portion of the net income for Chinese hospitals came from aggressive sales of antibiotics. This problem should surprise no one—the system gave powerful reimbursement incentives and hospitals responded.
Given these problems, how should we pay for antibiotics?
This is the key question posed to our DRIVE-AB research group. We’ve made some significant progress on it, but it’s a complex question. Wealthy countries like the US need to make it less expensive for companies to conduct research that sustains antimicrobial effectiveness. One model might look like the Orphan Drug Act, but for antibiotics. Significantly increased NIH (National Institutes of Health) grants for basic research are a clear winner. In later stages of research, government agencies like BARDA (US Office of Biomedical Advanced Research and Development Authority) step in to help promising antibiotics make it through clinical trials and FDA (Food and Drug Administration) approval. BARDA is doing an impressive job with antibiotics, and President Obama’s new budget gives more money to BARDA to expand their work. The open questions we are working on now is what happens after the FDA approves the drug. Traditionally, it has been the “price-volume” model, but many of us think a new form of reimbursement—delinkage—deserves a closer look. One delinkage idea is prizes…
Prizes?
Yes, like a $200 million dollar prize for FDA approval of a new antibiotic meeting an urgent, unmet medical need, rewarding the company but also preserving the drug from overmarketing and making sure it is available when needed. We’ve been exploring antibiotic prizes for several years and policymakers are starting to take notice. Just last week, an Op-Ed in the New York Times called for huge antibiotic prizes. Part of the work at DRIVE-AB is to execute research projects in order to get these incentives right.
 Methicillin-resistant Staphylococcus aureus, or MRSA, is a bacteria that is resistant to many antibiotics. In medical facilities, MRSA causes life-threatening bloodstream infections, pneumonia, and surgical site infections, according to the CDC. Image courtesy of National Institute of Allergy and Infectious Diseases (NIAID).
Methicillin-resistant Staphylococcus aureus, or MRSA, is a bacteria that is resistant to many antibiotics. In medical facilities, MRSA causes life-threatening bloodstream infections, pneumonia, and surgical site infections, according to the CDC. Image courtesy of National Institute of Allergy and Infectious Diseases (NIAID).
Obama’s new budget calls for greatly increased funding for antibiotic resistance. What are your thoughts?
The President’s budget is a huge, earth-shatteringly excellent proposal. Congress might quibble with some of the details, but this is a dangerous problem that demands urgent action. Our response must be measured in billions, not millions, of dollars.
You call for leadership by universities to solve these complex problems. Why universities?
Antimicrobial resistance is the ultimate interdisciplinary problem. Each discipline has a limited perspective on the problem; everyone has their special hammer and tools for the corresponding nail. Resistance is exactly the sort of problem that is too complicated, too interdisciplinary, to be solved by anyone other than academic researchers working together in universities. In fact, I think the very purpose of a modern research university is to tackle incredibly complex global problems like resistance.
You talk a lot about how antibiotic resistance is a global problem—that pathogens don’t respect borders—and in this month’s WHO Bulletin, you’re the co-author of an editorial calling for an international global framework.
Economically, antibiotic effectiveness is a global common pool resource. Think of it like a lake with fish in it. What we have now is everyone lives around the lake—all the countries of the world. Everyone can take the fish out, nobody’s thinking about maintaining the fish for the long term. Some people are fishing with poles, others with nets. A few are fishing with hand grenades. We’re just being ecologically stupid about how we’re handling this precious resource.
So, are we losing the war on bacteria right now?
I don’t like the term ‘war’ when talking about bacteria. Declaring war on bacteria is really declaring war on ourselves. Our lives are immeasurably entwined with commensal (helpful or harmless) bacteria. You don’t burn down a house to kill a spider. The proper goal is human health, in a long-term sustainable balance with everything in our environment, including bacteria. My research project is to use the law to restore this ecological balance, preventing us from returning to a pre-antibiotic era.
This story can also be found by visiting BU Research.