ENG Faculty Pivot to Fight COVID-19
By Liz Sheeley
“I’m glad I’m an engineer right now,” says Professor Joyce Wong (BME, MSE). “There are so many problems that need to be solved in this crisis and I can actually use my expertise to help.”
Wong, like many other engineers and researchers, is diving in to do what she can to mitigate the COVID-19 pandemic. Across the College of Engineering, professors are pivoting their research to tackle the many problems associated with the current pandemic.
These efforts are in addition to the first wave of help that gathered personal protective equipment (PPE) from now-closed labs and donated it to healthcare workers in Massachusetts.
Medical equipment to stop the spread of the virus
Wong started working on two projects after talking to her cousin, Dr. Steven Horng, an emergency medicine physician at Beth Israel Deaconess Medical Center (BIDMC).
“I started hearing about the PPE shortages from Steven, and then he started to tell me about more of the challenges healthcare workers are facing,” she says. “We’re getting close to the predicted peak of cases in Massachusetts, so I want to help out any way I can.”
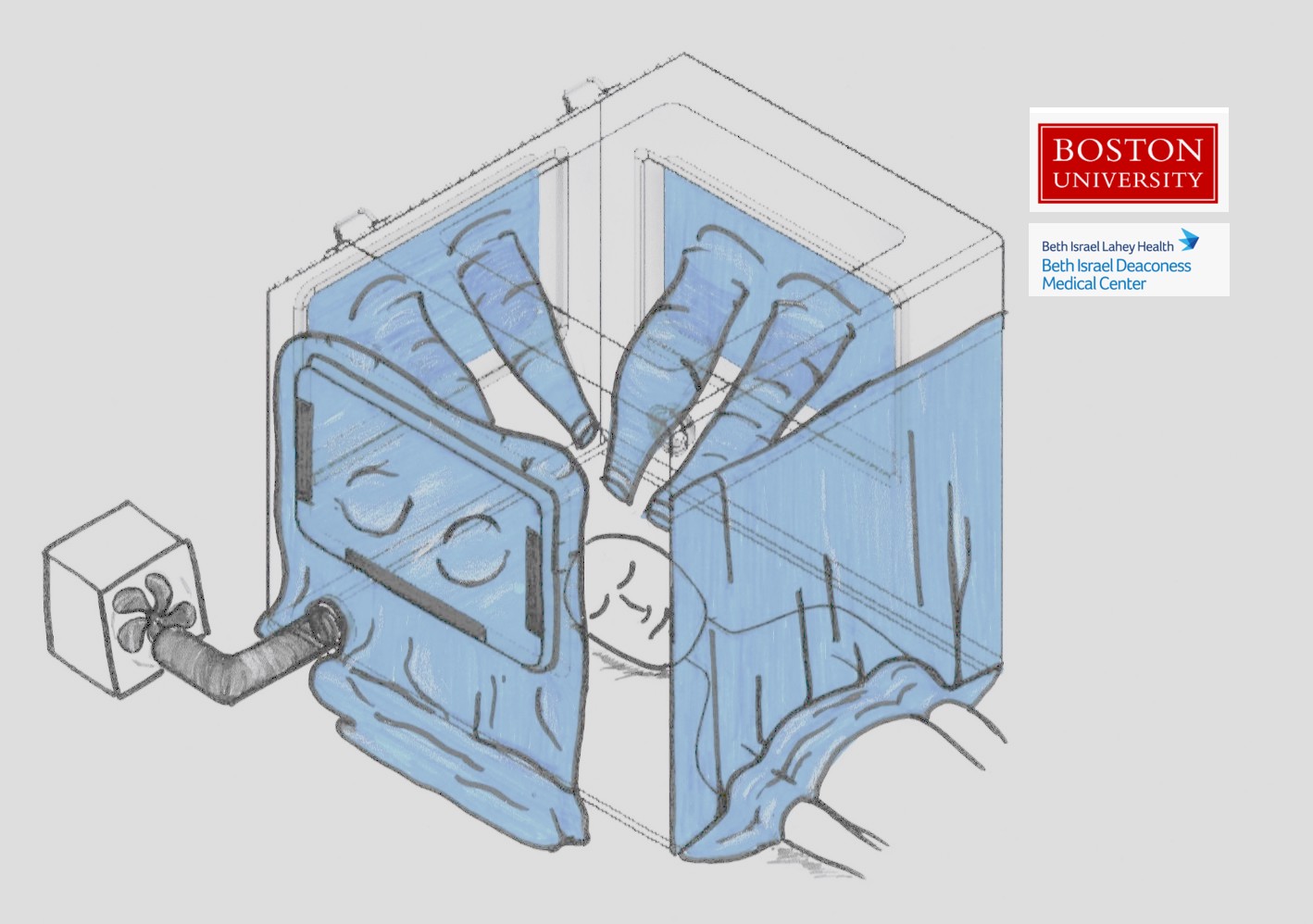
Both of Wong’s projects are collaborations with Master Lecturer Enrique Gutierrez-Wing (ME) and Associate Professor J. Gregory McDaniel (ME, MSE) and are aimed at keeping the virus contained. The first flips who is wearing the protective equipment from the healthcare workers to the patients. Her cousin saw a photo of an intubation box a Taiwanese doctor built, and together Horng and Wong thought that if they could expand on that idea and make the box a negative pressure chamber to isolate the source of the virus.
They’re aiming to design the respiratory isolation box with features to allow doctors to maintain the same standard of care with an added layer of protection. In addition to being a negative pressure chamber so that air doesn’t flow out of the box, it’s also being designed so that three people can work on one patient at a time, and they’re testing the dimensions to make sure healthcare workers can still use the specialized equipment they may need when intubating a COVID-19 patient. They have brought on Assistant Professor Emily Whiting (Computer Science) and Associate Professor Patricia Fabian (SPH) to help with the design.
The box is in the prototyping phase, and the researchers are working with Horng and his clinician colleagues, Henry Feldman, Cullen Jackson and Jason Maley, at BIDMC to test the design in their simulation lab. Feldman is a hospitalist who holds dual appointments in the Division of General Internal Medicine in the section of Hospital Medicine and in the Division of Clinical Informatics; Jackson is director of Quality, Safety & Innovation in Department of Anesthesia, Critical Care & Pain Medicine; and Maley is a clinical fellow in the Pulmonary and Critical Care Medicine Department.

The second collaboration is a 3D-printed bracket that will hold together an endotracheal tube and the respirator circuit it’s attached to. In normal use, these connections between the tube that’s inserted down the patient’s throat and the ventilator machine is loose, designed to be easily disconnected in case the patient needs to be moved to an emergency situation. Sometimes, however, the loose connection comes apart randomly, triggering an alarm that alerts a nurse to reconnect it.
But now, when that disconnection happens, the air coming out of the tube into the room is full of virus, putting anyone in the room at an unnecessarily high risk of infection. The bracket the team is developing easily clips into place to hold the tube and respirator hook-up together to prevent this from happening.
The bracket is 3D-printed in a material that can be medically grade sterilized, and the prototype is designed with rounded edges so it won’t rip latex gloves.
A different type of test
Professor Selim Ünlü (ECE, MSE, BME) is teaming up with long-time collaborator John Connor from the National Emerging Infectious Disease Laboratory (NEIDL) at BU and Professor Mehmet Toner from Harvard Medical School to develop rapid and reliable test for the SARS-Cov-2 virus that causes COVID-19.
The current tests look for viral RNA. Building on his previous research, Ünlü’s test is fundamentally different; it would count individual SARS-CoV-2 viruses using antibodies to capture the viruses on the sensor’s surface which can detect and count them.
The primary benefit of this approach is that it’s testing mechanism doesn’t require extensive sample preparation. Another benefit is a reduced chance of false negative results. Viruses can mutate, and the current tests rely on knowing specific genetic sequences of the virus to detect it. If the virus mutates within one of those sequences, the test could report a false negative (this happened during the 2014 Ebola outbreak). Another benefit of this test is that it gets closer to indicating that someone is infectious as it detects intact viruses rather than viral genetic material.
Ünlü also noted that his test would have a different supply chain that the existing test, leaving less prone to shortages than the current method.
Validating new tests
Professor and Director of the Precision Diagnostics Center Catherine Klapperich (BME, MSE) and her lab are helping by working to validate new types of SARS-CoV-2 tests. An extreme ramp up of testing is needed, but there are roadblocks and shortages of supplies to make that possible. New types of test must be validated and that takes time. Klapperich’s team is trying to speed that process up.
The Precision Diagnostics Center is taking on pre-clinical lab validation of newly developed tests, such as one coming out of a group from Harvard Medical School. Associate Professor Michael Springer’s lab is working on an RNA test that is faster than the standard one and has limited sample preparation.
Springer’s lab taught Klapperich’s how to perform their new assay, and her team has validated a version of his test for influenza using a bank of de-identified H1N1 patient samples from the 2009-2010 pandemic. Now with proof-of concept, Springer can tweak his assay to make it work for the SARS-CoV-2 virus. In the meantime, Klapperich is awaiting de-identified COVID-19 patient samples from collaborators at Boston Children’s Hospital that will be used to validate the next iteration of Springer’s assay and others.
Tracing potential infections
When patients test positive for the novel coronavirus, they need to quarantine for at least two weeks. That isolation is to stop the spread of the virus, and public health experts ask the infectious patients who they’ve been in contact with recently so they can alert those people that they might be infected. That process is called contact tracing and it’s how experts learn how infectious a virus is and how they trace it back to patient zero. But with hundreds of thousands of cases nationally, and thousands of new ones every day, traditional contact tracing is no longer practical.
Professor Ari Trachtenberg (ECE, Computer Science) along with Research Associate Professor Mayank Varia (Computer Science) and Professor Ran Canetti (Computer Science) are proposing an alert system that can replace standard contact tracing using smart phones.
The idea is to develop an app that people could opt into by downloading it and signing up. The app would leverage a short-range communication signal such as Bluetooth to constantly broadcast a signal to other nearby participating devices. Each device would have its own unique ID, and the app would record those IDs that it came into close contact with and keep a running list.
Participants could also agree to update their health status within the app after testing positive for the virus, and share this information with those they came in contact with. More widespread testing would enhance such a program by identifying infected people who don’t exhibit symptoms (current estimates indicate that could be 25 percent of those infected). One layer of security to keep people from marking themselves as positive without verification is to only have verified medical professionals be able to mark participants as positive after a test.
Steps would be taken to maintain participant privacy, such as new, random ID generation every so often. The IDs would also need to be too long to spoof, and the app would also only be available through public health agencies. Participation would also be completely voluntary and opt-in.
A system like this could help us get on top of the spread of this virus; it’s a solution that is easy to develop and launch. Researchers understand that security and privacy are top concerns with a system like this and know that preventing abuse and maintaining authenticity are completely necessary steps to take before launching such an app.
Improving nasal swabs
Because of the COVID-19 pandemic, the supply chain for the nasopharyngeal swabs used to collect patient samples for testing has not been able to keep up with demand. Seeking to find an alternative, pathologist Joel Henderson of BU’s School of Medicine and Boston Medical Center (BMC) reached out to BU’s College of Engineering for help. Jessie Song, a graduate student pursuing a PhD in biomedical engineering, answered the call.
Song, who does research with BU engineering faculty members Alice White and Mark Grinstaff, is an expert at using nanoscale 3D printing to create tissue scaffolds. She immediately saw the potential of using 3D printing to fabricate nasopharyngeal swabs. Working from prototype designs of nasopharyngeal swabs from the University of South Florida and Northwell Health in New York, Song selected a safe and sterilizable resin—often used to fabricate FDA-approved dental medical devices—and assembled the tools necessary to make several different prototypes. Within one week of receiving Henderson’s call for help, the first batch of nasopharyngeal swabs was printed overnight at BU’s Multiscale Laser Lithography laboratory.
“Jessie is a prime example of what makes BU a great place—it is the students,” Grinstaff says.
Henderson and his team are now evaluating the swabs Song fabricated to decide which designs they prefer and why, as well as how best to sterilize and package the swabs into kits.
Not only would this 3D printing approach, using new materials, skirt the supply chain issue that prompted Henderson’s call for help, it could also reduce the likelihood of false negative COVID-19 test results. Nasal swabs used for coronavirus tests must be pushed up a patient’s nose to collect a mucus sample from where the throat meets the back of the nose, which requires training and can be prone to user error. A swab made of a material that more easily collects mucus, increasing the chance of capturing a high-quality sample, would help reduce false negatives, the researchers say. Once an optimal swab design is identified, the team plans to conduct a clinical trial at BMC.