Blood Vessel-On-A-Chip Study Reveals Key Proteins’ Regulatory Role in Leaky Vessels
By Liz Sheeley
Blood vessels act as a transportation system, bringing and discarding molecules to and from each organ to keep the internal stability our bodies need. But during chronic disease, or even minor injury, blood vessels can be damaged enough to compromise vital organ function.
Professor Christopher Chen (BME, MSE), director of the Biological Design Center and associate faculty at the Wyss Institute at Harvard University, has now built a way to study blood vessel function in a way that closely mimics the real thing. Chen and his team created a 3-D blood vessel-on-a-chip model that can be used to understand what happens to vessels during injury on a molecular scale. “We hope to use these devices to learn more about how to keep vessels healthy, and about what goes awry in disease settings,” he said. Their model and findings are outlined in a paper published online in Proceedings of the National Academy of Sciences.
Scientists have long known that the endothelial cells that line the inside of blood vessels are the main regulators of the barrier in between the blood circulating within the vessels and the rest of the body. There are also supporting cells, called mural cells, which control vessel function, but simply have not been studied enough to understand their role in vessel barrier regulation.
When a part of the body is injured, the immune system kicks into high gear. Molecular signals are released that recruit certain cells and proteins to aid in the healing process. Those healing processes can cause inflammation, which can resolve itself after time, but not always. When the body sends out a signal that triggers inflammation, certain proteins are released that cause the blood vessel to lose its ability to act as a barrier. With chronic disease, such as liver cirrhosis, the blood vessels are constantly exposed to these proteins, and the loss of barrier function is prolonged. That constant disturbance can lead to significantly more harm, such as impaired blood flow to the injured area and ultimately tissue damage.
The 3-D blood vessel-on-a-chip has allowed Chen and the team to identify specific proteins that regulate vascular barrier function. The researchers studied three proteins–RhoA, Rac 1 and N-cadherin. It has already been well documented that during an inflammatory response, RhoA is activated, so this is where Chen started.
To study barrier function in the vessel-on-a-chip, the researchers introduced molecules that trigger an inflammatory response into the 3-D model. Then they could measure activity levels of specific proteins along with how penetrable the vessel became under inflammatory conditions. By measuring both the levels of activity of these three proteins and the vessel barrier function at the same time, they could gauge how each protein’s activity affected the barrier.
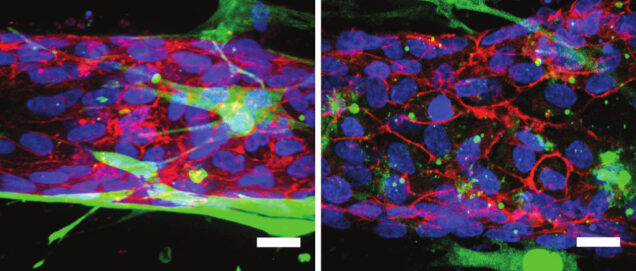
For example, once inflammation was triggered, RhoA’s activity levels increased, which resulted in a loss of barrier function; this was expected. To confirm their result, the researchers inhibited RhoA activity, and barrier function in the vessel was restored. However, when RhoA was targeted to activate only the mural cells, barrier function was lost.
Chen was able to show that a specific balance of these three proteins—RhoA, Rac 1 and N-cadherin—must be sustained in order to preserve the junction between endothelial cells and mural cells. Each one of these proteins plays a different role in maintaining vessel barrier function. Not only do they regulate the protective function endothelial cells provide, but they also affect mural cells—and furthermore regulate how both cell types interact with each other during inflammation. Previously, researchers had only been able to study how inflammation affects endothelial cells, and not how it affects mural cells. Their results suggest that leaky vessels are caused by a simple inflammatory response, and that they are not the result of complex signaling mechanisms that the literature currently deems the culprit.
Organs-on-a-chip are a cheap, efficient and accurate method to study what goes on in the body without using human test subjects. Now, with the 3-D blood vessel-on-a-chip, scientists in a laboratory can study cell-to-cell interactions in vasculature in the most lifelike setting yet.