A Hidden Pathway is Revealed
New Device Leads to Discovery That May Benefit Cancer Patients
by Liz Sheeley
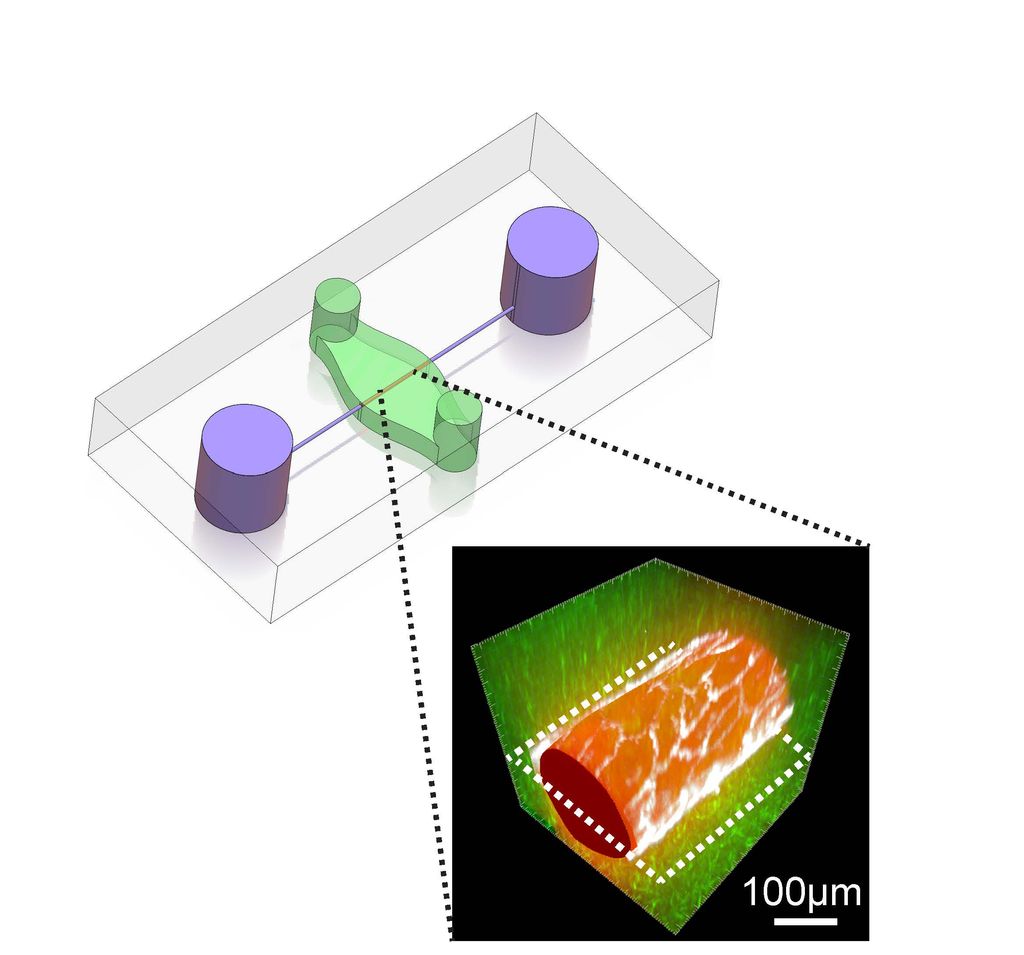
Two BME researchers were excited to use a new 3D blood vessel-on-a-chip that they developed in Professor Christopher Chen’s (BME, MSE) lab because it provided a perfect platform for studying the effects of mechanical forces of blood flow on vessels in life-like conditions. Their excitement was well founded—the research uncovered a previously unknown molecular pathway that elucidates how blood vessels maintain their integrity and could explain why cancer patients experience side effects from certain therapeutic drugs.

The question William Polacheck and Matthew Kutys were trying to answer was how mechanical forces affect blood vessel walls and why they leak. When blood flows through vessels, it exerts forces called shear stress onto their walls. “We knew there was a link between shear stress and barrier function,” said Polacheck. The chip enabled them to identify that link by allowing them to replicate the effects of blood flow and measure vessel leakiness, which was not previously possible. They soon noticed that the forces activated a protein signaling pathway called Notch that has been long known to play a major role in cellular behavior by turning genes on and off. But Polacheck and Kutys discovered that Notch also has another, previously unknown, function that could explain leaky vessels. “We were seeing that Notch was activated when we simulated blood flow, so we needed to connect Notch to barrier function to close the circle,” said Polacheck.
They discovered that physical forces on the blood vessel walls cause the Notch pathway to build a complex of proteins in the cell membrane and trigger a process that holds neighboring cells together, preventing fluid from leaking between them. Their work has been published in Nature.
Leaky vessels alone can lead to major health problems, and this new pathway may provide insights into how it occurs. In addition, some newer cancer therapeutics target the Notch pathway in an effort to block the overgrowth of cells. By blocking the entire Notch pathway, these drugs may also be inhibiting the heretofore unknown function of sealing blood vessel walls, causing them to leak. Indeed, these drugs have reported such side effects, and until now, clinicians have been at a loss to explain why this occurs. Polacheck and Kutys may have found the answer.
“Going forward, we hope to establish a better understanding of where and when this pathway contributes to health and disease,” said Chen. “We also will continue our efforts to build new cell culture platforms that can be used to mimic and study important disease processes.”