CMLD Researchers Publish Paper on “Remodeling” Natural Products in Nature Chemistry
CMLD-BU researchers Bradley Balthaser, Meghan Maloney, Aaron Beeler, John Porco & John Snyder, in a paper published in the journal Nature Chemistry [23 OCTOBER 2011 | DOI: 10.1038/NCHEM.1178], present a new approach to accessing new, biorelevant structures by “remodeling” natural products. In this case, they demonstrate how the natural product derivative fumagillol can been remodeled to access a collection of new molecules using highly efficient chemical reactions.
“Overall, these studies should pave the way for work to identify pharmacological tools for use in CNS research, oncology, and as anti-infective agents,” said John A. Porco, Jr., professor of chemistry at Boston University. “These studies also will enable future studies to remodel additional natural product scaffolds to access novel therapeutic agents.”
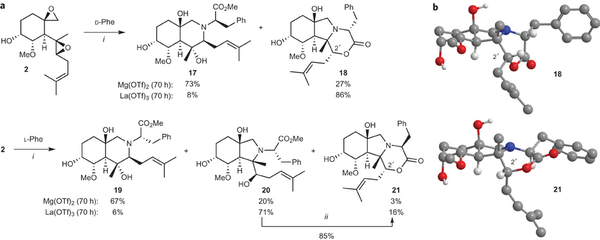
In the search for novel biologically active molecules, DOS strategies break through the limitation of traditional library synthesis by sampling new chemical space. Many natural products can be regarded as useful starting points for DOS, wherein stereochemically rich core structures may be reorganized into chemotypes that are distinctly different from the parent structure. Ideally, to be suited to library applications, such transformations should be general and involve few steps.
With this objective in mind, Porco and colleagues including Professor John Snyder and postdoctoral fellow Dr. Brad Balthaser successfully remodeled the highly oxygenated natural product fumagillol in several ways using a reaction-discovery-based approach. In reactions with amines, excellent selectivity in a bis-epoxide opening/cyclization sequence was obtained using the appropriate metals catalysts forming either perhydroisoindole or perhydroisoquinoline products. Perhydroisoindoles were further remodelled to other complex structures including novel benzoxazepines.
The Center for Chemical Methodology and Library Development at Boston University (CMLD-BU) is a research center funded by the National Institute of General Medical Sciences ( NIGMS ) focused on the discovery of new methodologies to produce novel chemical libraries of unprecedented complexity for biological screening. The goal of the CMLD-BU is to explore and expand the diversity of small-molecule libraries by creating general, useful protocols for stereocontrolled synthesis. See the CMLD-BU’s website for more information.